Key Points
Question
What is the status of reporting and representation of racial/ethnic groups in landmark trials leading to US Food and Drug Administration (FDA) approval of oncology drugs?
Findings
In 230 trials leading to FDA oncology drug approvals over the past decade, race was reported in only 145 (63%) trials. Compared with whites (98% of expected proportion), blacks (22% of expected proportion) and Hispanics (44% of expected proportion) were underrepresented in these trials relative to their proportion among the US cancer population.
Meaning
Suboptimal race reporting and representation (especially in blacks and Hispanics) occurs regularly in landmark oncology trials and increased efforts are needed to enhance minority representation and eliminate these disparities.
This database study assesses the frequency of race reporting and proportional race representation in trials that support FDA oncology drug approvals.
Abstract
Importance
Representative racial/ethnic participation in research, especially in clinical trials that establish standards of care, is necessary to minimize disparities in outcomes and to uphold societal equity in health care.
Objective
To evaluate the frequency of race reporting and proportional race representation in trials supporting US Food and Drug Administration (FDA) oncology drug approvals.
Design, Setting, and Participants
Database study of all reported trials supporting FDA oncology drug approvals granted between July 2008 and June 2018. Primary reports of trials were obtained from PubMed and ClinicalTrials.gov. Food and Drug Administration approvals were identified using the FDA archives. The US population-based cancer estimates by race were calculated using National Cancer Institute–Surveillance, Epidemiology, and End Results and US Census databases.
Main Outcomes and Measures
Primary outcomes were the proportion of trials reporting race and the proportion of patients by race participating in trials. Secondary outcomes included race subgroup analyses reporting and gaps between race proportion in trials and the US population. Descriptive statistics, Fisher exact, and χ2 tests were used to analyze the data. Proportions and odds ratios (OR) with 95% CIs were reported.
Results
Among 230 trials with a total of 112 293 participants, 145 (63.0%) reported on at least 1 race, 18 (7.8%) documented the 4 major races in the United States (white, Asian, black, and Hispanic), and 58 (25.2%) reported race subgroup analyses. Reporting on white, Asian, black, and Hispanic races was included in 144 (62.6%), 110 (47.8%), 88 (38.2%), and 23 (10.0%) trials, respectively. Between July 2008 and June 2013 vs July 2013 and June 2018, the number of trials reporting race (45 [56.6%] vs 100 [67.1%]; OR, 1.63; 95% CI, 0.93-2.87; P = .09) and race subgroup analysis (13 [16.1%] vs 45 [30.2%]; OR, 2.26, 95% CI, 1.16-4.67; P = .03) changed minimally and varied across races. Whites, Asians, blacks, and Hispanics represented 76.3%, 18.3%, 3.1% and 6.1% of trial participants, respectively, and the proportion for each race enrolled over time changed nominally (blacks, 3.6% vs 2.9% and Hispanics, 5.3% vs 6.7%) from July 2008 to June 2013 vs July 2013 to June 2018. Compared with their proportion of US cancer incidence, blacks (22% of expected) and Hispanics (44% of expected) were underrepresented compared with whites (98% of expected) and Asians (438% of expected).
Conclusions and Relevance
Race and race subgroup analysis reporting occurs infrequently, and black and Hispanic races are consistently underrepresented compared with their burden of cancer incidence in landmark trials that led to FDA oncology drug approvals. Enhanced minority engagement is needed in trials to ensure the validity of results and reliable benefits to all.
Introduction
Racial disparities in health care and biomedical research are multidimensional and originate in factors that span society and medicine.1,2,3,4 Racial diversity in clinical trials serves as a metric of societal equality and access to health care, while also allowing assessment of biologic differences that may determine differential efficacy of drugs. This is particularly important in racially pluralistic societies such as the United States because studies have demonstrated survival differences from cancer by race, even after controlling for socioeconomic and treatment differences.5,6,7 Unfortunately, trial populations frequently do not represent the population they are intended to emulate. Hispanic and black patients with cancer have lower enrollment rates on clinical trials than whites in the United States.8 This disparity prevents minorities from sharing the benefits of scientific advances and makes trial results less generalizable. In addition, racially associated genetic variation can be an important determinant of drug metabolism and response.9 Well-designed trials need to be able to assess racial differences in metabolism and biomarker prevalence that affect clinical outcomes.10 Organizations such as the National Institutes of Health (NIH) and the US Food and Drug Administration (FDA) have recognized the importance of this issue and have taken steps to increase reporting and representation of minorities in research; however, it remains unclear how these efforts have affected racial diversity in oncology trials.11,12
Given the importance of racial diversity in ensuring the validity, equality, and scientific rigor of research, our aim was to determine how well it was reflected and accounted for in pivotal drug trials that established new standards of care in oncology. We reviewed clinical trials that led to all FDA approvals for oncology drugs over the past decade (2008-2018) to determine whether race was reported and whether these trials represented the racial diversity of the United States. We hypothesized that many trials did not report race, did not evaluate the association between race and outcome, and were not representative of the patient population with the disease studied that was ultimately treated.
Methods
Study Cohort
We systematically reviewed FDA drug approvals granted from July 2008 through June 2018 from the FDA archives.13 Trials that supported these approvals were then identified using PubMed and the National Institutes of Health (NIH) trials registry (ClinicalTrials.gov).
Only 9 (3.9%) of 230 trials reported Hispanic ethnicity as distinct from race. Therefore, for brevity, we use the term race to refer to both race and ethnicity. Likewise, because only 5 (2.2%) trials reported on Native American race and only 13 Native Americans reportedly participated in these trials, limiting any meaningful interpretation, all analyses were restricted to 4 major race categories within the United States: white, Asian, black, and Hispanic. Trials characteristics and race reporting were abstracted from journal articles (primary reports) or the most recent abstract and/or presentation if a study was unpublished. When multiple trials supported FDA approval, each trial was included in the analysis. Race was considered reported for an approval if at least 1 of the trials supporting the approval reported race. Subgroup analyses for primary end points based on race were also recorded.
The US population-based cancer incidence and mortality data were collected from the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results (SEER) Program database (SEER 18: 2000-2015).14 Age-standardized incidence and mortality rates adjusted to the year 2000 standard US population by race were used to calculate estimated new cancer cases and deaths in 2018 (using US Census population estimates15) as a measure of proportion of cancer burden by race. This study of published reports and publicly available data was exempt from institutional review board approval and patient written informed consent requirements by the Common Rule and our institutions’ policies.
Statistical Analysis and Outcome Measures
The proportion of trials reporting race and subgroup analyses was calculated among all reported trials. The proportion of patients of a specific race who participated in trials was calculated after censoring trials that did not report any information on that race. To measure enrollment disparity for each race in absolute terms, we calculated the enrollment incidence disparity (EID) as the absolute difference between the proportion of patients of a particular race among trial participants in cancer drug approval studies and the estimated proportion of patients of that particular race diagnosed with a specific cancer type among the US population. We also calculated the enrollment mortality disparity (EMD) as the absolute difference between the proportion of patients of a particular race among trial participants and the estimated proportion of patients of that particular race who died from a specific cancer type among the US population. Similarly, to measure enrollment disparity for each race in relative terms, we calculated the enrollment incidence ratio (EIR) and enrollment mortality ratio (EMR) as the proportion of patients of a particular race among trial participants in approval studies divided by the estimated proportion of patients of that particular race diagnosed or dying from a specific cancer type, respectively, among the US population. Standard descriptive statistics were used and proportions were summarized with their 95% CIs.16 Group comparisons were performed using the χ2 or Fisher exact test, as appropriate, and odds ratios (ORs) with 95% CIs. P values were 2-sided and considered statistically significant when unadjusted P < .05.
Results
Baseline Approval and Trial Characteristics
We identified 204 FDA drug approvals (150 [73.5%] full and 54 [26.4%] accelerated) for patients with solid tumors (68.1%) and hematological cancers (31.9%) over the past decade (Figure 1). These approvals were based on 232 clinical trials and covered 108 drugs across diverse indications (eTable 1, eTable 2, and eFigure 1 in the Supplement). Baseline characteristics of approvals and trials are shown in the Table. Most approvals were not based on randomized studies (n = 147 [72.1%]) and commonly involved targeted therapy (n = 94 [46.1%]) or immunotherapy (n = 39 [19.1%]). Trials leading to FDA approval were mostly phase 3 (n = 148 [65.1%]), randomized (n = 161 [69.4%]), and industry sponsored (n = 225 [97.0%]). Two trials, associated with 1 approval, were excluded from subsequent analyses because no report was available.
Figure 1. Study Schema and Race Reporting in FDA Approvals in Hematology/Oncology.
A, The US Food and Drug Administration (FDA) approved 108 drugs in 204 distinct approvals based on 232 clinical trials. B, Drug approvals increased over the past 10 years (2008 to 2018). Less than 50% of the trials leading to these approvals reported on other than white races. C, The absolute number of patients of races other than white who participated in pivotal trials leading to FDA approval was considerably low.
aFDA hematology/oncology (cancer) approvals and safety notifications.13
bData obtained from principal publication of trials. A total of 227 reports were retrieved using PubMed (1 approval was based on 2 trials had no available reports at data lock on December 1, 2018. It was excluded from the analyses).
cHispanic origin was reported both as race and as ethnicity across different studies and has been combined for analyses in the present study. Therefore, Hispanics and non-Hispanics are not mutually exclusive from whites, blacks, Asians, and Native Americans.
d2008 and 2018 Approval data limited to second-half and first-half of years, respectively.
eTotal populations specified per race enrolled across 144 trials reporting on race expressed to scale. The size of sphere represents the absolute number of patients.
Table. Characteristics of FDA Approvals/Trials for Hematology/Oncology, July 2008 to June 2018a.
| Characteristics | No. (%) |
|---|---|
| Mechanism of Action for Drug Approval | |
| Targeted therapy | 94 (46.1) |
| Tyrosine kinase inhibition | 61 (29.9) |
| Epigenetic modulation | 10 (4.9) |
| Antibody drug conjugates | 7 (3.4) |
| Cell-cycle inhibition | 6 (2.9) |
| DNA repair mechanisms | 6 (2.9) |
| Others | 4 (2.0) |
| Immunotherapy | 39 (19.1) |
| Immune checkpoint inhibition | 32 (15.7) |
| Others | 7 (3.4) |
| Monoclonal antibodiesb | 22 (10.8) |
| Antiangiogenesis | 12 (5.9) |
| Cytotoxic chemotherapy | 18 (8.8) |
| Others | 19 (9.3) |
| Approval Characteristics | |
| Year of approval | |
| July 2008-June 2013 | 67 (32.8) |
| July 2013-June 2018 | 137 (67.2) |
| Type of approval | |
| Full | 150 (73.5) |
| Accelerated | 54 (26.5) |
| Approval based on No. of trials | |
| 1 | 182 (89.2) |
| 2 or 3 | 22 (10.8) |
| Approval based on randomized trialc | |
| Yes | 57 (27.9) |
| No | 147 (72.1) |
| Drug approval combined with other | |
| Yes | 65 (31.9) |
| Alone | 139 (68.1) |
| Specific Disease For Approval | |
| Solid tumor oncology | 139 (68.1) |
| Non–small cell lung cancer | 35 (17.2) |
| Melanoma | 18 (8.8) |
| Renal cell/urothelial carcinoma | 16 (7.8) |
| Breast cancer | 15 (7.4) |
| Colorectal cancer | 9 (4.4) |
| Prostate cancer | 9 (4.4) |
| Others | 51 (25.0) |
| Hematology | 65 (31.9) |
| Multiple myeloma | 12 (5.9) |
| Leukemia | |
| Acute | 12 (5.9) |
| Chronic lymphocytic | 11 (5.4) |
| Chronic myeloid | 10 (4.9) |
| Lymphoma | |
| Hodgkin | 6 (2.9) |
| Follicular | 6 (2.9) |
| Others | 22 (10.8) |
| Trial Characteristics | |
| Arms on trialc | |
| Single arm | 47 (20.3) |
| Multiple arm | 185 (79.7) |
| Phase of triald,e | |
| 1/2 | 83 (35.9) |
| 3 | 148 (65.1) |
| Sponsor of trialc | |
| Academia and/or academia and industry | 7 (3.0) |
| Industry | 225 (97.0) |
| Randomized triald | 161 (69.4) |
| Placebo-controlled trial | 73 (31.5) |
| Drug approval combined with other | |
| Yes | 65 (31.9) |
| Alone | 139 (68.1) |
| Size of triald | |
| ≤100 | 34 (14.7) |
| 100-500 | 114 (49.1) |
| >500 | 84 (36.2) |
Abbreviation: FDA, US Food and Drug Administration.
204 Total approvals, 232 total trials, 108 approved drugs.
Anti–programmed cell death 1, anti–programmed cell death ligand 1, and anti-cytotoxic T-lymphocyte antigen-4 antibodies were considered as immunotherapies (not monoclonal antibodies).
All multistudy approvals with at least 1 study that was randomized were included in randomized category.
All proportions for trials were calculated using total number of trials (N = 232).
Phase of trial was not specified for 1 study.
Race Reporting and Subgroup Analyses in Trials
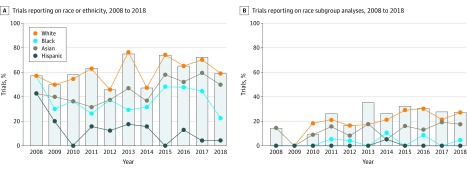
Of 230 reported trials, 145 (63.0%) reported on at least 1 race as a baseline characteristic, while 85 (36.9%) had no mention of race (Figure 1A and Figure 2A). White, Asian, black, or Hispanic race was reported in 144 (62.6%), 110 (47.8%), 88 (38.2%), and 23 (10.0%) trials, respectively (Figure 1A and Figure 2A). Only 18 (7.8%) trials reported on all 4 races. Race reporting changed minimally from July 2008 to June 2013 (45 [55.6%] trials) vs July 2013 to June 2018 (100 [67.1%] trials) (OR, 1.63; 95% CI, 0.93-2.87; P = .09) (eFigure 2 in the Supplement). This change in reporting varied by race (Figure 2A and eTable 3 in the Supplement). Studies involving targeted therapy (77 of 106 [72.6%]) and chemotherapy (15 of 19 [78.9%]) were more likely than studies of other drug mechanisms to report race. Similarly, solid tumor trials (109 of 156 [69.9%]) were more likely to report race than hematology trials (36 of 74 [48.6%]). No other factors were associated with reporting of race (eTable 5 in the Supplement).
Figure 2. Race Reporting and Race-Related Subgroup Analyses in Trials for FDA Approval of Hematology/Oncology Drugs.
A, The charts show a trend over past 10 years in trials leading to US Food and Drug Administration (FDA) drug approvals with regard to the proportion of trials reporting on race/ethnicity (A) and the proportion of trials reporting on subgroup analysis pertaining to race/ethnicity (B). Although the sharp increase in the number of trials leading to FDA-approved drugs has been increasing over time, there has been a relatively smaller improvement in reporting of race or subgroup analyses based on race, and these reporting rates vary significantly between races. The overall reporting of race and race subgroup analyses remains low even in 2018.
Of the 145 trials that reported on race, 58 (40.0%) reported at least 1 subgroup analysis by race (Figure 2B). Among all reported trials, a subgroup result for white, Asian, black, and Hispanic races was reported in 49 (21.3%), 32 (13.9%), 5 (2.2%), and 1 (0.4%) trial, respectively (Figure 2B). The number of trials reporting race subgroup analyses increased from July 2008 to June 2013 vs July 2013 to June 2018 (13 [16.1%] vs 45 [30.2%]; OR, 2.26; 95% CI,1.16-4.67; P = .03) and varied by race (eFigure 2, Figure 2B, and eTable 4 in the Supplement). Phase 3 (44 [47.8%] of 92) and solid tumor (50 [45.9%] of 109) studies were more likely to report subgroup analyses involving race (eTable 6 in the Supplement). Subgroup analyses for primary end points were reported infrequently for races other than white, and none of the trials explicated a rationale for carrying out these analyses (eFigure 3 in the Supplement).
Racial Representation in Trials
A total of 112 293 patients participated in the 230 reported trials that led to FDA oncology approvals in the last decade. The 145 (63.0%) trials that reported race enrolled 70 201 (62.5%) patients, of which 53 342, 10 285, 1513, 630, and 13 were white, Asian, black, Hispanic, and Native American, respectively (Figure 1C). Among trials that reported on patients of the assessed races, the overall proportion for whites, Asians, blacks, and Hispanics enrolled in trials was 76.3%, 18.3%, 3.1%, and 6.1%, respectively (Figure 3). In trials that documented all 4 races, these proportions were 82.3%, 7.5%, 4.5%, and 5.1%, respectively (eFigure 5 in the Supplement). The relative proportion of patients from each race enrolled over time remained fairly constant (Figure 3A and eFigure 4 in the Supplement), although there was a small increase in Hispanic patients during the second half of the decade (July 2013-June 2018 vs July 2008-June 2013) (6.7% vs 5.3%; OR, 1.27; 95% CI, 1.08-1.49; P = .004) and a decline in black patients (2.9% vs 3.6%; OR, 0.81; 95% CI, 0.73-0.90; P < .001) (eTable 7 in the Supplement). To adjust for global participation in trials, we performed a sensitivity analysis, excluding indications where age-adjusted incidence in Asia was higher than in America and found that these proportions continued to be low at 81.6%, 13.7%, 4.0%, and 5.5% for whites, Asians, blacks, and Hispanics, respectively (eFigure 8 in the Supplement) (Asia was selected for sensitivity analysis given the overrepresentation of Asians relative to the cancer incidence of Asians in the United States).
Figure 3. Differences in Incidence, Mortality, and Enrollment in Clinical Trials Leading to FDA Oncology Drug Approvals vs US Population With Cancer.
A, Proportion of different races in trials for US Food and Drug Administration (FDA) approval from 2008 to 2018. B, Relative proportion of different races (pertaining to incidence and mortality) among patients with cancer in the United States was estimated using the Surveillance, Epidemiology, and End Results database and compared with trial participants in FDA approval trials between July 2008 and June 2018. Representation of black and Hispanic patients in pivotal FDA approval studies was low from 2008 to 2018.
Trial characteristics compared by race are summarized in eTable 8 in the Supplement. In July 2008 to June 2013 vs July 2013 to June 2018, the proportion of whites (OR, 0.78; 95% CI, 0.75-0.81; P < .001) and blacks (OR, 0.81; 95% CI, 0.73-0.90; P < .001) entering trials decreased, whereas Hispanic race proportion increased (OR, 1.27; 95% CI, 1.08-1.49; P = .004). Whites were more likely to participate in phase 3 (OR, 1.14; 95% CI, 1.08-1.20; P < .001), randomized (OR, 1.36; 95% CI, 1.29-2.88; P < .001), and multiarm trials (OR, 1.37; 95% CI, 1.28-2.92; P < .001) compared with blacks (phase 3: OR, 0.63; 95% CI, 0.55-0.72; randomized: OR, 0.57; 95% CI, 0.50-0.66; multiarm: OR, 0.73; 95% CI, 0.60-0.88), Asians (phase 3: OR, 0.71; 95% CI, 0.53-0.75; randomized: OR, 0.61; 95% CI, 0.58-0.65; multiarm: OR, 0.55; 95% CI, 0.48-0.60), and Hispanics (phase 3: OR, 0.69; 95% CI, 0.54-0.87; randomized: OR, 0.62; 95% CI, 0.48-0.80; multiarm: OR, 0.47; 95% CI, 0.35-0.61), who were more likely to participate in phase 2, nonrandomized, and single-arm trials (eTable 8 in the Supplement).
Racial Disparity Between Trial and US Population
In an attempt to establish the external validity of trials for the US population (because they were used for FDA approvals), we compared the proportions of patients of each race among trial participants vs the US population.
Black and Hispanic patients were consistently underrepresented compared with their expected proportion based on cancer incidence and mortality in the United States, whereas Asian patients appeared to be overrepresented, and white patients had enrollment that nearly matched their expected proportion (Figure 3B and eFigure 6 in the Supplement). For all cancers together, the EID and EIR were unfavorable for blacks (−11.3% and 0.22) and Hispanics (−7.8% and 0.44) compared with whites (−1.7% and 0.98) and Asians (+14.1% and 4.38) (eFigure 6 in the Supplement). Similarly, the EMD and EMR were unfavorable for blacks (−13.6% and EMR, 0.19) and Hispanics (−6.3% and 0.48) compared with whites (0.6% and 1.01) and Asians (14.6% and 4.95) (eFigure 6 in the Supplement). The results were consistent for almost all cancer types, with consistent underrepresentation of black and Hispanic races compared with white and Asian races, although the degree of disparity varied across tumor types (Figure 4 and eFigure 7 in the Supplement).
Figure 4. Relative Differences in Incidence, Mortality, and Enrollment in Clinical Trials Leading to FDA Drug Approval for Specific Indications.
The proportion of different races represented in trials for US Food and Drug Administration (FDA) approval of oncology drugs for specific indications compared with the proportion of different races (pertaining to incidence [I] and mortality [M]) among patients with a specific cancer vs the US population estimated using data from the Surveillance, Epidemiology, and End Results database. Analysis was restricted to indications where trials (>1) reported on white, Asian, and black races. There is consistent underrepresentation of black and Hispanic races in pivotal FDA approval studies between July 2008 and June 2018. CML indicates chronic myeloid leukemia; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma.
Discussion
From July 2008 to June 2018, reporting of race and diversity of race representation was poor in oncology trials that led to FDA oncology drug approvals. Race was not reported in more than one-third of these trials (n = 85). Among trials that documented race, representation of blacks and Hispanics was very low relative to US cancer population estimates. Although prior studies demonstrated underrepresentation of racial/ethnic minorities in clinical trials, these studies were restricted to NCI-funded trials and did not necessarily affect patient care.17,18,19 Our study highlights the presence of these disparities, even among pivotal trials informing new drug approvals for the US populace with cancer. With efforts such as the Affordable Care Act attempting to improve access to standards of care, we demonstrate a pressing need to improve representation in cancer research.20
Multiple efforts have recognized the importance of encouraging diversity in clinical trials and have prioritized enhanced reporting on racial subgroups, strategies to encourage participation, and transparency of subgroup data in trials (eTable 9 in the Supplement).12,20,21,22,23,24,25,26,27,28 The FDA recommends accurate collection and reporting of race/ethnicity and expects sponsors to enroll patients who reflect clinically relevant populations (eTable 9 in the Supplement).22 Despite this progressive stance, the mechanisms to enforce these recommendations and their influence on improving minority representation is unclear. National Institutes of Health guidelines mandate proportional racial representation in NIH-funded clinical research; however, because most trials that lead to drug approvals are funded by industry, broader reaching efforts are needed.12,25
Underrepresented populations face numerous barriers to clinical trial participation.3,29 The perception of trials differs based on historic influences. One group may consider trials “cutting edge,” whereas another may have reservations about being a “guinea pig.” The Tuskegee syphilis study exemplifies lasting distrust toward research that may affect participation.30 We noted significant differences in the types of trials entered by each race. White patients entered larger, randomized, phase 3, multiarm trials, and minorities entered smaller, nonrandomized, single-arm trials. These findings raise concerns that certain recruitment methods may affect minority races differently.
Other barriers, such as distance to treatment, lack of transportation, and lower income also disproportionately affect minorities.31,32 In 2017, median US household income was highest among Asians ($81 331), followed by whites ($68 145), Hispanics ($50 486), and blacks ($40 258).33 These income patterns closely mirror the disparities in trial participation seen in our study. Indeed, income has been identified as an independent predictor of oncology trial participation.34,35 Although median household income and socioeconomic status may explain part of our findings, the reasons for participation in trials are complex. Without a multipronged approach, including community outreach programs, greater minority representation in medical schools, reimbursement for indirect health care costs, and a dialogue with minorities about the barriers they experience, this situation is unlikely to improve.
Although improving trial diversity is a noble social goal, we also need improved diversity for scientific reasons. The rates of key driver mutations, such as epidermal growth factor receptor mutations in lung cancer, vary dramatically across races.10 Black patients appear to have greater intratumor genetic heterogeneity and worse survival than white patients despite enrollment in similar trials with uniform characteristics.6,36 This finding suggests an underlying biological connotation to the social construct of race and indicates a need to understand this complexity. Proportional representation ensures that results are generalizable to the patients treated with approved agents and facilitates subgroup analyses of drug metabolism and biomarker differences. The emphasis on the rare molecular subset is ever-increasing, and a lack of trial diversity will impede the ability to move into the era of precision medicine. The findings reported herein, that relatively few trials have examined racial subgroups and none have provided a rationale for these analyses, are notable. Although subgroup analyses are often underpowered, limited racial representation complicates them further and can have negative consequences for patients in the real world.37 Proportional representation and examination of the efficacy of an intervention within minority subgroups should only be conducted based on a priori hypotheses with a strong rationale.37
Although desirable, it is challenging and expensive to prospectively facilitate adequate race participation. Conducting smaller follow-up studies, collecting phase 4 data on an underrepresented population, and depositing trial information on publicly accessible portals, such as Project Data Sphere, can facilitate exploration of race-specific analyses.38,39
Limitations
Although our study highlights a critical issue, it should be interpreted within the context of several limitations. Although we extrapolated our analysis to the United States, global recruitment may have skewed the different racial/ethnic mixes. Similarly, the incidence estimates are not granular enough to account for molecular subsets within diverse races, such as epidermal growth factor receptor mutations in lung cancer. However, the principle of external validity for these trials in the US population still stands because eventually FDA approval permits use of drugs by the US populace based on global studies. Finally, it is possible that the rates of minority enrollment of studies that did not report race tended to be low; if so, the true underlying rate of enrollment for minorities in FDA trials may be even lower than we have reported, which suggests that our estimates of disparity are actually conservative. Lastly, we acknowledge that increasing representation, although necessary, must be accompanied by exploration of the contributions of biological factors underlying racial differences. We also recognize that social, economic, and environmental factors are equally important factors in propagating disparities in cancer care.
Conclusions
Despite efforts to eliminate health care disparities, gaps in race reporting and disparate representation persist in oncology trials. Black and Hispanic patients are consistently underrepresented relative to the US population in trials used for FDA cancer drug approvals. Reducing cancer care disparities is a multidimensional task that extends beyond trial accrual and reporting, and there is a pressing need for affirmative policies, dedicated disparity research, and social/regulatory interventions to increase representation of minority groups in cancer research. Given the growing racial and ethnic pluralism in society, it is a scientific and ethical imperative to ensure that our research reflects and benefits all.
eTable 1. List of drugs approved by FDA for oncology between July 2008 and June 2018
eTable 2. Hematological malignancies and solid tumors for which drugs were approved by FDA between July 2008 and June 2018
eFigure 1. Common hematological malignancies (Panel A) and solid tumors (Panel B) for which drugs were approved by FDA between July 2008 and June 2018
eFigure 2. Comparison of growth of trials that led to FDA oncology approvals through the last 10 years with reporting of race (Panel A) and race subgroup analyses (Panel B)
eTable 3. Comparison of rate at which race was reported in clinical trials leading to FDA oncology approvals over past decade
eTable 4. Comparison of rate at which race subgroup analyses was reported in clinical trials leading to FDA oncology approvals over past decade
eTable 5. Factors associated with race reporting among clinical trials leading to FDA oncology approvals over past decade
eTable 6. Factors associated with reporting of subgroup analyses for race (race subgroup) among clinical trials leading to FDA oncology approvals over past decade
eFigure 3. Hazard ratios (HRs) for progression-free survival (PFS) (Panel A) and overall survival (OS) (Panel B) reported in trials for patients with reported subgroup analyses based on race
eFigure 4. Comparison of proportion of patients enrolled on FDA approval studies by races through the last 10 years
eFigure 5. Proportion of different races in trials for FDA approval over the years in all trials (solid lines) and in trials with complete race reporting (dashed lines)
eTable 7. Comparison of relative proportion of patients by race (specific race vs. others) enrolled in clinical trials leading to FDA oncology approvals over past decade
eTable 8. Summary of patient race/ethnicity stratified by trial characteristics
eFigure 6. Enrollment to incidence disparity and ratio (EID and EMD) and Enrollment to mortality disparity and ratio (EIR and EMR) for all races and all cancers (Panel A, B and C)
eFigure 7. Enrollment incidence disparity (EID), enrollment incidence ratio (EIR), enrollment mortality disparity (EMD), and enrollment mortality ratio (EMR)
eTable 9. Summary of efforts to address racial disparities in research
eFigure 8. Proportion of races reported in trials for FDA approval over the years (Sensitivity analysis)
eReferences.
References
- 1.US Census Bureau Demographic turning points. population projections for the United States: 2020. to 2060. https://www.census.gov/content/dam/Census/newsroom/press-kits/2018/jsm/jsm-presentation-pop-projections.pdf. Accessed July 9, 2019.
- 2.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):-. doi: 10.1093/jnci/94.5.334 [DOI] [PubMed] [Google Scholar]
- 3.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242. doi: 10.1002/cncr.23157 [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) Committee on Cancer Research Among Minorities and the Medically Underserved The Unequal Burden of Cancer. Washington, D.C.: National Academies Press; 1999, doi: 10.17226/6377. [DOI] [PubMed] [Google Scholar]
- 5.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program in the United States, 1990 to 2010. JAMA Oncol. 2015;1(1):88-96. doi: 10.1001/jamaoncol.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984-992. doi: 10.1093/jnci/djp175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahal BA, Berman RA, Taplin ME, Huang FW. Prostate cancer–specific mortality across Gleason scores in black vs nonblack men. JAMA. 2018;320(23):2479-2481. doi: 10.1001/jama.2018.11716 [DOI] [PubMed] [Google Scholar]
- 8.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 9.Kobayakawa M, Kojima Y. Tegafur/gimeracil/oteracil (S-1) approved for the treatment of advanced gastric cancer in adults when given in combination with cisplatin: a review comparing it with other fluoropyrimidine-based therapies. Onco Targets Ther. 2011;4:193-201. doi: 10.2147/OTT.S19059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma BB, Hui EP, Mok TS. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol. 2010;11(1):75-84. doi: 10.1016/S1470-2045(09)70160-3 [DOI] [PubMed] [Google Scholar]
- 11.Smedley B, Stith A, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003, doi: 10.1001/jama.290.18.2487-b. [DOI] [PubMed] [Google Scholar]
- 12.Freedman LS, Simon R, Foulkes MA, et al. Inclusion of women and minorities in clinical trials and the NIH Revitalization Act of 1993—the perspective of NIH clinical trialists. Control Clin Trials. 1995;16(5):277-285. doi: 10.1016/0197-2456(95)00048-8 [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food & Drug Administration Hematology/oncology (cancer) approvals & safety notifications. 2018. https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm279174.htm. Accessed January 2, 2019.
- 14.National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat database: incidence—SEER 9 Regs Research Data, Nov 2018 Sub (1975-2016) <Katrina/Rita Population Adjustment>—Linked To County—Total U.S., 1969-2017 Counties. Surveillance Research Program, released April 2019, based on the November 2018 submission. https://seer.cancer.gov/. Accessed July 11, 2019.
- 15.US Census Bureau US Census. 2018. https://www.census.gov/. Accessed January 2, 2019.
- 16.Agresti A, Coull BA. Approximate is better than “Exact” for interval estimation of binomial proportions. Am Stat. 1998;52(2):119-126. doi: 10.1080/00031305.1998.10480550 [DOI] [Google Scholar]
- 17.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109-2117. doi: 10.1200/JCO.2002.08.056 [DOI] [PubMed] [Google Scholar]
- 18.Chen MS Jr, Lara PN, Dang JHT, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120(suppl 7):1091-1096. doi: 10.1002/cncr.28575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang HH, Wang X, Stinchcombe TE, et al. Enrollment trends and disparity among patients with lung cancer in national clinical trials, 1990 to 2012. J Clin Oncol. 2016;34(33):3992-3999. doi: 10.1200/JCO.2016.67.7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sealy-Jefferson S, Vickers J, Elam A, Wilson MR. Racial and ethnic health disparities and the Affordable Care Act: a status update. J Racial Ethn Health Disparities. 2015;2(4):583-588. doi: 10.1007/s40615-015-0113-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Food & Drug Administration FDASIA section 907: inclusion of demographic subgroups in clinical trials. https://www.fda.gov/RegulatoryInformation/LawsEnforcedbyFDA/SignificantAmendmentstotheFDCAct/FDASIA/ucm389100.htm. Accessed January 2, 2019.
- 22.U.S. Food & Drug Administration Collection of race and ethnicity data in clinical trials: guidance for industry and food and drug administration staff. https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126396.pdf. Accessed January 2, 2019.
- 23.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793-795. doi: 10.1056/NEJMp1500523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United States National Institute of Health All of us research program. 2015. https://allofus.nih.gov/. Accessed January 2, 2019.
- 25.National Institutes of Health NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2017. https://grants.nih.gov/grants/funding/women_min/guidelines.htm. Accessed January 2, 2019.
- 26.Congress.gov. S.1880—Minority Health and Health Disparities Research and Education Act of 2000. https://www.congress.gov/bill/106th-congress/senate-bill/1880. Accessed April 1, 2019.
- 27.National Institutes of Health National Institute on Minority Health and Health Disparities system-level health services and policy research on health disparities (R01). 2016. https://grants.nih.gov/grants/guide/rfa-files/RFA-MD-15-001.html. Accessed April 1, 2019.
- 28.National Institutes of Health National Institute of Minority Health and Health Disparities community based participatory research program. 2009. https://grants.nih.gov/grants/guide/rfa-files/rfa-md-09-004.html. Accessed April 1, 2019.
- 29.Unger JM, Cook E, Tai E, Bleyer A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book. 2016;35(36):185-198. doi: 10.1200/EDBK_156686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockwell DH, Yobs AR, Moore MB Jr. The Tuskegee study of untreated syphilis. Arch Intern Med. 1964;114(6):792-798. doi: 10.1001/archinte.1964.03860120104011 [DOI] [PubMed] [Google Scholar]
- 31.Guidry JJ, Aday LA, Zhang D, Winn RJ. Transportation as a barrier to cancer treatment. Cancer Pract. 1997;5(6):361-366. [PubMed] [Google Scholar]
- 32.Silver D, Blustein J, Weitzman BC. Transportation to clinic: findings from a pilot clinic-based survey of low-income suburbanites. J Immigr Minor Health. 2012;14(2):350-355. doi: 10.1007/s10903-010-9410-0 [DOI] [PubMed] [Google Scholar]
- 33.US Census Bureau. Real median household income by race and Hispanic origin: 1967 to 2017. current population survey, 1968-2018 annual social and economic supplements. 2018. https://www.census.gov/content/dam/Census/library/visualizations/2018/demo/p60-263/figure1.pdf. Accessed January 2, 2019.
- 34.Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139. doi: 10.1001/jamaoncol.2015.3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542. doi: 10.1200/JCO.2012.45.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keenan T, Moy B, Mroz EA, et al. Comparison of the genomic landscape between primary breast cancer in African American versus white women and the association of racial differences with tumor recurrence. J Clin Oncol. 2015;33(31):3621-3627. doi: 10.1200/JCO.2015.62.2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine—reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189-2194. doi: 10.1056/NEJMsr077003 [DOI] [PubMed] [Google Scholar]
- 38.Taylor AL, Wright JT Jr. Should ethnicity serve as the basis for clinical trial design? importance of race/ethnicity in clinical trials: lessons from the African-American Heart Failure Trial (A-HeFT), the African-American Study of Kidney Disease and Hypertension (AASK), and the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Circulation. 2005;112(23):3654-3660. doi: 10.1161/CIRCULATIONAHA.105.540443 [DOI] [PubMed] [Google Scholar]
- 39.Project Data Sphere https://www.projectdatasphere.org/projectdatasphere/html/home. Accessed February 2, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. List of drugs approved by FDA for oncology between July 2008 and June 2018
eTable 2. Hematological malignancies and solid tumors for which drugs were approved by FDA between July 2008 and June 2018
eFigure 1. Common hematological malignancies (Panel A) and solid tumors (Panel B) for which drugs were approved by FDA between July 2008 and June 2018
eFigure 2. Comparison of growth of trials that led to FDA oncology approvals through the last 10 years with reporting of race (Panel A) and race subgroup analyses (Panel B)
eTable 3. Comparison of rate at which race was reported in clinical trials leading to FDA oncology approvals over past decade
eTable 4. Comparison of rate at which race subgroup analyses was reported in clinical trials leading to FDA oncology approvals over past decade
eTable 5. Factors associated with race reporting among clinical trials leading to FDA oncology approvals over past decade
eTable 6. Factors associated with reporting of subgroup analyses for race (race subgroup) among clinical trials leading to FDA oncology approvals over past decade
eFigure 3. Hazard ratios (HRs) for progression-free survival (PFS) (Panel A) and overall survival (OS) (Panel B) reported in trials for patients with reported subgroup analyses based on race
eFigure 4. Comparison of proportion of patients enrolled on FDA approval studies by races through the last 10 years
eFigure 5. Proportion of different races in trials for FDA approval over the years in all trials (solid lines) and in trials with complete race reporting (dashed lines)
eTable 7. Comparison of relative proportion of patients by race (specific race vs. others) enrolled in clinical trials leading to FDA oncology approvals over past decade
eTable 8. Summary of patient race/ethnicity stratified by trial characteristics
eFigure 6. Enrollment to incidence disparity and ratio (EID and EMD) and Enrollment to mortality disparity and ratio (EIR and EMR) for all races and all cancers (Panel A, B and C)
eFigure 7. Enrollment incidence disparity (EID), enrollment incidence ratio (EIR), enrollment mortality disparity (EMD), and enrollment mortality ratio (EMR)
eTable 9. Summary of efforts to address racial disparities in research
eFigure 8. Proportion of races reported in trials for FDA approval over the years (Sensitivity analysis)
eReferences.