Abstract
Paradormancy of fruit trees occurs in summer and autumn when signals from adjacent organs stimulate buds to develop slowly. This stage has received less attention that the other stages of dormancy, and the underlying mechanism remains uncharacterized. Early defoliation in late summer and early autumn is usually followed by out-of-season blooming in pear (Pyrus spp.), which substantially decreases the number of buds the following spring and negatively affects fruit production. This early bud flush is an example of paradormancy release. Here, we determined that flower bud auxin content is stable after defoliation; however, polar distribution of the pear (Pyrus pyrifolia) PIN-FORMED auxin efflux carrier 1b (PpyPIN1b) implied that auxin tends to be exported from buds. Transcriptome analysis of floral buds after artificial defoliation revealed changes in auxin metabolism, transport, and signal transduction pathways. Exogenous application of a high concentration of the auxin analog 1-naphthaleneacetic acid (300 mg/L) suppressed PpyPIN1b expression and its protein accumulation in the cell membrane, likely leading to decreased auxin efflux from buds, which hindered flower bud sprouting. Furthermore, carbohydrates and additional hormones also influenced out-of-season flowering. Our results indicate that defoliation-induced auxin efflux from buds accelerates bud paradormancy release. This differs from release of apical-dominance-related lateral bud paradormancy after the apex is removed. Our findings and proposed model further elucidate the mechanism underlying paradormancy and will help researchers to develop methods for inhibiting early defoliation-induced out-of-season bud sprouting.
Early defoliation promotes auxin efflux from buds to release paradormancy in autumn, inducing out-of-season flowering, which differs from release of apical-dominance-related lateral bud paradormancy.
Introduction
Bud dormancy, which refers to the bud inactive state caused by environmental conditions, bud internal changes, and exogenous signals, can be further divided into ecodormancy, endodormancy, and paradormancy (Lang et al., 1987; Gomez-Roldan et al., 2008; Umehara et al., 2008). Bud paradormancy is a temporary dormancy controlled by biochemical signals from other organs or tissues (e.g. apex, leaf, and bud scale) (Lang et al., 1987). Apical dominance likely plays a primary role in the paradormancy of current-year lateral buds in woody plants and lateral buds in herbaceous plants (Cline and Deppong, 1999; Costa and Ramina, 2014; Barbier et al., 2019). Many fruit trees have a paradormancy period in the summer and autumn. During this stage, the shoots stop elongating and there are no sizable changes in appearance, but the bud physiological differentiation associated with a transition from the vegetative to reproductive phase starts in mid-summer (Banno et al., 1986; Forshey and Elfving, 1989; Tromp, 2000). Subsequently, floral organ primordia develop in an orderly manner and buds mature gradually (Costa and Ramina, 2014). Although the morphological differentiation of flower buds is almost complete in late autumn, the buds generally do not sprout until the next season.
Recently, out-of-season blooming of pear in autumn has been a common occurrence almost every year in southern China. This abnormal blooming was induced by abnormal defoliation mainly caused by leaf diseases (Zhao et al., 2011; Huang et al., 2015). Flowering in autumn is a common phenomenon in several fruit tree species, including apple (Malus domestica), peach (Prunus persica), and plum (Prunus salicina Lindl.) (Wang and Wang, 2009). Similar phenomena have been observed worldwide (Ge et al., 2011). As buds are in the paradormant state in autumn, the unusual autumn flowering is related to paradormancy release. The fruit resulting from autumn flowering will drop from trees prematurely as the season progresses and the temperature decreases, resulting in decreases in the number of flower buds and the fruit yield in the following year (Zhao et al., 2011; Zhang et al., 2016). Therefore, the maintenance of paradormancy in autumn is important for fruit production in the following year. The mechanism underlying the unusual flowering in autumn in response to early defoliation remains unclear. Elucidating this mechanism will clarify the paradormancy process.
Previous studies about paradormancy mostly focused on apical depressions on lateral buds and branching (apical dominance) (Lang et al., 1987; Costa and Ramina, 2014; Barbier et al., 2019). The apical buds control the release and outgrowth of axillary buds, which are cooperatively regulated by diverse hormones, carbohydrates, and other signals (Domagalska and Leyser, 2011; Barbier et al., 2019). More specifically, auxin from apical tissues is a key regulator of lateral bud paradormancy (Faust et al., 1997), but it prevents their outgrowth without entering lateral buds (Hall and Hillman, 1975; Prasad et al., 1993; Booker et al., 2003). Two major models have been proposed to explain how auxin regulates apical dominance. The first is the competition model, in which auxin canalization from the bud to the stem promotes lateral bud break (Bangerth, 1989; Li and Bangerth, 1999; Bennett et al., 2006, 2016a; Prusinkiewicz et al., 2009). The polar transport of auxin synthesized in the shoot apical meristem to the stem increases the stem auxin concentration, which decreases the capacity of the stem to accept auxin from buds and inhibits the export of auxin from lateral buds. After the shoot tip is removed, the auxin flow from the apical tissue is cut off, which is followed by a decrease in the stem auxin content, making the axillary bud a stronger auxin source than the stem (Prusinkiewicz et al., 2009; Walker and Bennett, 2018). The establishment of the source–sink relationship between lateral buds and the stem accelerates the export of auxin from the lateral buds via the efflux carrier protein PIN, thereby releasing lateral buds from their dormant state. In the second proposed model, apically derived auxin regulates lateral bud release through second messengers such as strigolactone (SL) and cytokinin (CK) (Domagalska and Leyser, 2011; Müller and Leyser, 2011; Rameau et al., 2015). Auxin stimulates SL synthesis and decreases CK levels. SL represses bud growth (Gomez-Roldan et al., 2008; Umehara et al., 2008), whereas CK has the opposite effect (Dun et al., 2012; Müller et al., 2015). Auxin positively regulates the expression of the carotenoid cleavage dioxygenase genes MORE AXILLARY GROWTH 3 (MAX3) and MAX4 to increase SL levels to inhibit axillary bud outgrowth in Arabidopsis thaliana (Arabidopsis) (Sorefan et al., 2003) as well as in garden pea (Pisum sativum), rice (Oryza sativa), and petunia (Petunia hybrida) (Foo et al., 2005; Johnson et al., 2006; Arite et al., 2007; Simons et al., 2007; Brewer et al., 2009). SL negatively regulates bud outgrowth through the local inhibition of axillary bud break by inducing the production of the branching inhibitor BRANCHED1 (BRC1), a Teosinte branched1/Cycloidea/Proliferating cell factor (TCP) transcription factor, within buds as well as through the systematic inhibition of branching by hindering PIN-mediated auxin transport (Bennett et al., 2006, 2016b; Aguilar-Martínez et al., 2007; Prusinkiewicz et al., 2009; Crawford et al., 2010; Braun et al., 2012; Guan et al., 2012; Shinohara et al., 2013; Seale et al., 2017; Zhang et al., 2020). In contrast, auxin inhibits CK biosynthesis and induces CK degradation by disrupting the expression of the biosynthesis rate-limiting enzyme-encoding ISOPENTENYL TRANSFERASE (IPT) and the oxidase-encoding CK OXIDASE 2 (CKX2) in the nodal stem; the resulting decrease in the CK level restricts bud outgrowth (Tanaka et al., 2006; Shimizu-Sato et al., 2009). CK activates lateral buds by suppressing BRC1 in pea, rice, and Arabidopsis (Finlayson, 2007; Minakuchi et al., 2010; Braun et al., 2012; Dun et al., 2012). It also activates buds by promoting the accumulation and polar distribution of the PIN auxin efflux proteins in the plasma membrane of xylem parenchyma cells to induce auxin export from buds for branching (Kalousek et al., 2010; Waldie and Leyser, 2018).
Apical dominance is not the only factor regulating bud paradormancy. Auxin flow-regulated paradormancy involves the apical bud as well as other organs. Active organs tend to export auxin, which inhibits the efflux of auxin from other organs, thereby limiting the growth of the latter, a phenomenon known as auxin-transport autoinhibition (Bangerth, 1989; Prusinkiewicz et al., 2009). For example, in citrus (Citrus reticulata × Citrus sinensis) and olive (Olea europaea cv. Barnea), there is a strong flow of auxin from the fruits to the stem, which impedes auxin efflux from the axillary bud (Haim et al., 2020). Growing fruits induce inflorescence arrest by restricting auxin transport from the inflorescence apex (Goetz et al., 2021). Regarding early defoliation-induced bud sprouting, similar to fruits, the leaves may be involved in auxin canalization to maintain bud paradormancy, which differs from apical dominance. In addition to being regulated by hormones, bud paradormancy is also influenced by several flowering regulators, including FLOWERING LOCUS T (FT), TERMINAL FLOWER1 (TFL1), and LIKE-AP1 (LAP1) (Maurya et al., 2020a; Singh et al., 2021; Andre et al., 2022). FT and LAP1 positively regulate terminal and lateral bud growth, while TFL1 works contrary (Bohlenius et al., 2006; Hanano and Goto, 2011; Iwata et al., 2012; Azeez et al., 2014; Maurya et al., 2020b; Zhu et al., 2020). Short days and low temperatures induce the expression of TFL1 but reduce the LAP1, while both genes antagonistically control branching (Azeez et al., 2014; Miskolczi et al., 2019; Maurya et al., 2020a, 2020b; Singh et al., 2021). Furthermore, LAP1 also suppresses the expression of BRC1, which interacts with and antagonizes FT to promote growth cessation (Maurya et al., 2020b).
Apical dominance release occurs in lateral buds after the apical bud is removed, whereas paradormancy release after defoliation in rosaceous fruit trees occurs in buds from different types of shoots. Some rosaceous fruit trees (e.g. apple and pear) have two typical shoots, namely the long shoots and the spurs (Huet, 1973; Wilkie et al., 2008). A long current-year shoot can bear an apical bud and several lateral buds. The spurs have shortened internodes and form apical buds. Thus, early defoliation-induced autumn flowering or bud sprouting occurs in lateral buds as well as apical buds. Furthermore, bud sprouting after defoliation is predominantly influenced by the presence of leaves rather than apical organs. Compounds or signals from the leaves might regulate bud paradormancy. Therefore, whether leaves influence auxin distribution and flow to regulate early defoliation-induced out-of-season bud sprouting should be investigated.
Apical dominance and auxin-transport autoinhibition are involved in polar auxin transport (PAT), suggesting that auxin canalization may be critical for paradormancy release. We speculated that auxin canalization is controlled more by leaves rather than by apical dominance in early defoliation-induced bud paradormancy release. In this study, we revealed that auxin canalization contributes to paradormancy release, resulting in defoliation-induced out-of-season flowering. Defoliation turns buds into auxin sources, rather than stems, with the auxin efflux from buds promoting bud paradormancy release. Moreover, a transcriptome analysis of the spur flower buds after defoliation indicated that auxin has a key regulatory role in the early defoliation-induced paradormancy release. Thus, we propose a model for paradormancy release that involves the altered auxin distribution induced by early defoliation as well as strategies for preventing early defoliation-induced out-of-season bud sprouting.
Results
Development of “Cuiguan” pear spur flower buds under natural conditions
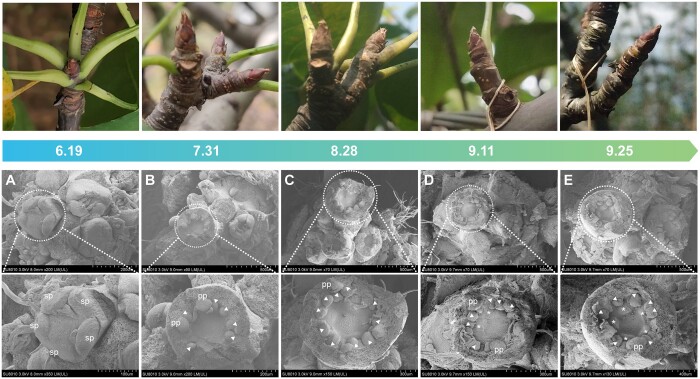
To clarify the bud morphological changes during paradormancy, we examined the development of paradormant spur flower buds under natural conditions. Buds begin to differentiate physiologically as the shoot meristem transitions from the vegetative to reproductive phase in early May. On the basis of scanning electron microscopy (SEM), the inflorescence primordium was detected in early June and multiple flower primordia were observed afterward. In mid-June (Figure 1A), five sepal primordia swelled on each flower primordium. Five petal primordia (small globules) formed medially to the sepal primordia, which was followed by the emergence of a ring of globular stamen primordia medial to the petal primordia in late July (Figure 1B). In late August (Figure 1C), the second whorl of stamen primordia appeared. Five pistil primordia formed a bulge on the inner side of the second whorl of stamen primordia from mid-to-late September (Figure 1, D and E). Therefore, flower buds had all of the floral organ primordia in late September.
Figure 1.
“Cuiguan” pear spur flower bud development under natural conditions in 2019. Photographs and scanning electron micrographs of spur flower buds on June 19 (A), July 31 (B), August 28 (C), September 11 (D), and September 25 (E). The bottom panel for each time-point is a magnified image of one of the flower buds in the upper panel. sp, sepal primordium; pp, petal primordium; triangles, stamen primordium; and asterisks, pistil primordium.
Defoliation advanced the development of spur flower bud to induce its break in autumn
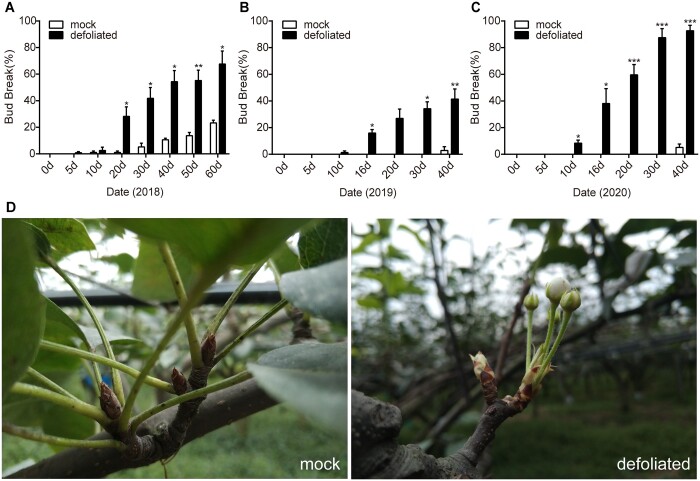
Under natural conditions, buds usually sprouted within a few days after early defoliation (Supplemental Figure S1A). To simulate the early defoliation in August (Supplemental Figure S1B), we removed approximately 70% of the leaves of the pear trees (Supplemental Figure S1C) in 2018, 2019, and 2020. The bud break rate was calculated for 40 days after defoliation. We observed that the bud break rate increased after the leaves were removed. Additionally, there were significant differences in the bud break rates between the defoliation and control treatments from 20, 16, and 10 days post-treatment in 2018, 2019, and 2020, respectively (Figure 2, A–C). The defoliation treatment substantially promoted bud release (Figure 2D), with the bud break rate exceeding 80% in 2020. These results indicated defoliation-induced spur flower bud break.
Figure 2.
Early defoliation induced “Cuiguan” pear spur flower bud break in autumn. A–C, Bud break rate after defoliation in 2018 (A), 2019 (B), and 2020 (C). D, Representative images of spur bud break on mock control and defoliated trees on day 20. Error bars indicate the SEs of three biological replicates and asterisks indicate significant differences between the mock control and defoliated trees (Student’s t test). *P < 0.05, **P < 0.01, ***P < 0.001.
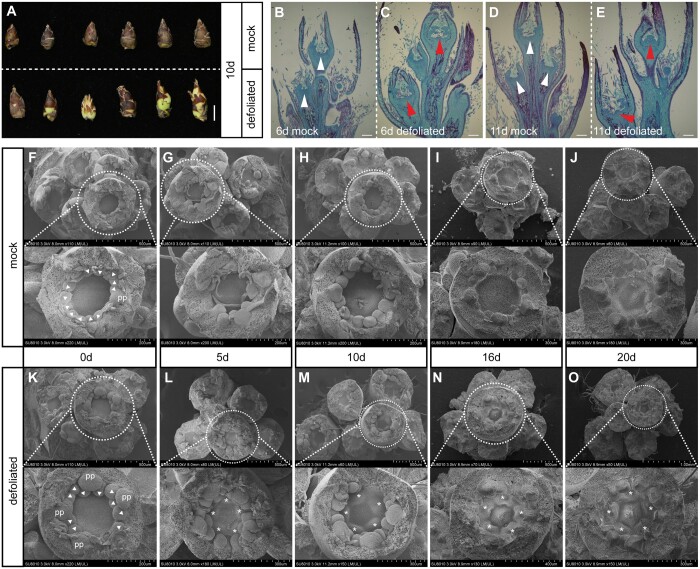
To determine the influence of defoliation on flower bud morphological development, we performed an anatomical examination. Some flower buds sprouted 10 days after defoliation (Figures 2C and 3A), whereas there were no visible changes in the control flower buds (Figure 3A). In the vertical section of the flower bud, pistil primordia were detected 6 days after defoliation (Figure 3, B and C), but they were more evident on day 10 (Figure 3, D and E). The SEM observation revealed the presence of five petal primordia and two whorls of stamen primordia before defoliation (Figure 3, F and K). Since 5 days after defoliation, pistil primordia emerged in the samples from defoliated trees (Figure 3, L–O); pistil primordia were undetectable in the control samples (Figure 3, G–I), even on day 20 (Figure 3J), which is consistent with the buds under natural conditions (Figure 1C). These results demonstrated that defoliation advanced the development of flower buds and further promoted flower bud break.
Figure 3.
Defoliation accelerated flower bud development. A, Photographs of dormant flower buds from mock control trees (top) and sprouted flower buds in different states from defoliated trees (bottom) at 10 days after defoliation in 2019. B–E, Vertical sections of flower buds collected at 6 days (B, C) and 11 days (D, E) after defoliation from mock control trees and defoliated trees in 2018. Red arrowheads indicate the pistil primordia of buds after defoliation. White arrowheads indicate the center of flower primordia without the pistil primordia of buds in the mock control group. F–O, Scanning electron micrographs of fruit spur flower buds from mock control trees (F–J) and defoliated trees (K–O) at 0, 5, 10, 16, and 20 days after defoliation in 2019. The central floral primordium is labeled with a white dashed line, with magnified images of the petal primordia, stamen primordia, and pistil primordia (bottom). Scale bars, 5 mm (A) and 200 μm (B–E). pp, petal primordium; triangles, stamen primordium; and asterisks, pistil primordium.
Altered auxin distribution in the bud and stem after defoliation
After the apical bud is removed, the axillary bud is released from apical dominance by the auxin transport canalization from the bud to the stem because the auxin level is lower in the stem than in the bud. We analyzed the auxin content in the buds and stems connecting spur buds to a branch during the autumn-flowering period. There were no obvious changes in the auxin content of spur flower buds according to the liquid chromatography–mass spectrometry (LC–MS) analysis (Figure 4, A and B). We further conducted an immunofluorescence-based localization experiment to examine slight auxin content changes (Supplemental Figure S2A). The fluorescence of auxin was slightly weaker in defoliated flower buds than in the mock control flower buds from day 5 after defoliation. Subsequently, the fluorescence intensity of indole acetic acid (IAA) was semi-quantified, using nuclear fluorescence intensity as an internal reference (Supplemental Figure S2B). The changes in the ratio were consistent with the fluorescence intensity revealed by the confocal microscopy image, indicative of a slight but not substantial decrease in the flower bud IAA level after defoliation.
Figure 4.
Changes in the IAA content in spur flower buds and stems at the base of flower buds and immunofluorescence localization of PpyPIN1b in flower buds at 7 days after defoliation. A, Flower bud IAA contents at 0, 5, 10, and 20 days after defoliation in 2018. B, Flower bud IAA contents at 0, 1, 3, 5, 7, and 10 days after defoliation in 2019. C, Stem IAA contents at 0, 3, 7, and 10 days after defoliation. **P < 0.01. D, Left and right panels, respectively, present flower buds from the mock control and defoliated groups at 7 days after defoliation. The fluorescence in the upper images indicates the nuclear and PpyPIN1b fluorescence of flower buds. The fluorescence in the lower magnified images represents the PpyPIN1b protein immunofluorescence localization signal. White arrowheads indicate the cell membrane in which PpyPIN1b was distributed.
Although there was no significant fluctuation in the bud auxin content, we observed that the stem IAA content increased after defoliation and was considerably higher than that of the control on days 7 and 10 after defoliation (Figure 4C). To explore whether these observations were related to auxin efflux from buds, we examined the distribution of the auxin efflux transporter PIN-FORMED auxin efflux carrier 1b (PpyPIN1b) in flower buds via immunofluorescence (Figure 4D). Confocal microscopy images revealed that PpyPIN1b tended to be localized in the one side of the cell membrane from day 3 after defoliation; however, in the mock control group, there was non-polar PpyPIN1b localization in the cell membrane (Figure 4D). Hence, IAA efflux from flower buds appeared to increase after defoliation.
Thus, defoliation does not significantly modulate the bud auxin content, but it accelerates the auxin efflux from buds to promote bud break.
RNA sequencing analysis and functional annotation of differentially expressed genes during bud paradormancy release after defoliation
We investigated the transcriptional changes in defoliation-induced paradormancy release, with a particular focus on the genes related to auxin and other hormones that interact with auxin. Flower bud break started 10 days after defoliation (August 5, 2020) (Figure 3A and Supplemental Figure S3A). Thus, we constructed 36 RNA sequencing (RNA-seq) libraries using flower buds stripped of bud scales (Supplemental Figure S4) at six time-points (0, 1, 3, 5, 7, and 10 days after defoliation) and obtained 1,795.1 million raw sequencing reads. The 8,831 identified differentially expressed genes (DEGs) were subjected to a pair-wise comparison of time-points (not including day 0) to investigate the overall changes in gene expression during defoliation-induced flowering (Supplemental Figure S3B). Day 5 had the most DEGs (6,754; 3,209 upregulated and 3,545 downregulated genes), followed by day 7 (4,775; 2,243 upregulated and 2,532 downregulated genes). Day 1 had the fewest DEGs (269).
To investigate the main altered pathways during defoliation-induced autumn flowering, we conducted a Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. The top 15 significantly enriched KEGG pathways among the upregulated (Supplemental Figure S3C) and downregulated (Supplemental Figure S3D) DEGs in each pair-wise comparison included “photosynthesis,” “starch and sucrose metabolism,” “plant hormone signal transduction,” “cytoskeleton proteins,” and “chromosome and associated proteins.”
We further explored the metabolic changes during defoliation-induced autumn flowering on the basis of a MapMan analysis (Figure 5A). Autumn flowering was divided into three stages according to the metabolic changes at various time-points. Days 1 and 3 after defoliation were included in stage 1, which comprised upregulated genes associated with the “light response” and “photorespiration.” Days 5 and 7 after defoliation were included in the second stage, during which major changes in energy metabolism (e.g. light reactions and sugar metabolism) and hormone activities occurred. The light reaction pathways were induced, especially those related to photosystem II (PSII) activities (Supplemental Figure S5A). Additionally, the genes annotated with “starch-sucrose” and “minor carbohydrate (CHO)” were related to sugar metabolism (i.e. starch/sucrose synthesis or catalysis) (Supplemental Figure S5B), suggesting they may help generate sugars for flower bud development (Patrick et al., 2013). The expression levels of genes involved in the “cell cycle” were upregulated. Moreover, the genes associated with “hormone action” were likely involved in the various changes to different hormones. Day 10 was included in stage 3, in which the DEGs were related to “hormone action” and the “cell cycle,” implying they might mediate flower bud growth and development. The enrichment analysis results indicated that several metabolic and signal transduction pathways (e.g. energy metabolism and hormone pathways) are involved in defoliation-induced flowering.
Figure 5.
Analyses of the metabolic activities and the expression of key DEGs involved in auxin metabolism and signaling pathways during defoliation-induced paradormancy release. A, MapMan analysis of the metabolic activities associated with DEGs in three defoliation-induced paradormancy release stages. B, Relative expression profiles of genes involved in auxin biosynthesis, storage, catabolism, transport, and signaling pathways. TAA1, tryptophan aminotransferase; YUCCA, indole-3-pyruvate monooxygenase; CYP79B2/CYP79B3, tryptophan N-monooxygenase; NIT, deaminated glutathione amidase; AMI1, amidase; TDC, tyrosine decarboxylase; ALDH, acetaldehyde dehydrogenase; UGT, UDP-glycosyltransferase; TGW6, indole-3-acetic acid-glucose hydrolase; IAMT1, indole-3-acetate O-methyltransferase; MES17, methylesterase; ILR1/IAR3/ILL, IAA-amino acid hydrolase; GH3, indole-3-acetic acid-amido synthetase; DAO, 2-oxoglutarate-dependent dioxygenase; UGT74D1, UDP-glycosyltransferase; AUX/IAA, auxin-responsive protein; PIN, auxin efflux carrier component; ABCB, ABC transporter B family member; and AUX/LAX, auxin influx transporter.
To further verify the changes in DEGs, eight DEGs were selected for a reverse-transcription quantitative PCR (RT-qPCR) analysis (Supplemental Figure S6). The gene-specific RT-qPCR primer pairs used in this study are listed in Supplemental Table S1. The RT-qPCR and RNA-seq data were consistent, which confirmed the reliability of the transcriptome analysis.
Transcriptional changes in auxin metabolism and signaling genes in buds after defoliation
To investigate the role of hormones, we analyzed 117 hormone-related genes that were differentially expressed during defoliation-induced flowering (Supplemental Figure S7). Among these genes, 41, 24, 16, 10, and 4 were associated with auxin metabolism and signaling, abscisic acid (ABA), CK, ethylene (ETH), and SL metabolism, respectively. Additionally, nine brassinosteroid (BR)-related genes, five jasmonic acid (JA)-related genes, four gibberellin (GA)-related genes, and four salicylic acid (SA)-related genes were identified.
To clarify the effects of auxin on defoliation-induced paradormancy release, we analyzed the transcriptional changes to genes associated with auxin metabolism, transport, and signaling (Figure 5B). The tryptophan pathway is the main auxin synthesis pathway. The expression levels of the key genes in this pathway were substantially upregulated at 3–5 days after defoliation (Figure 5B), including the genes encoding the IAA synthesis rate-limiting enzyme (PpyYUCCA), tryptophan aminotransferase (PpyTAA1), and acetaldehyde dehydrogenase (PpyALDH). In addition to the increased expression of auxin synthesis-related genes, the expression of PpyMES genes was also upregulated (Figure 5B). These genes encode proteins that regulate the conversion between free IAA and the stored or conjugated forms of IAA (IAA-Pro, IAA-Gluc, MeIAA, and indole-3-butyric acid (IBA)). Moreover, GH3 catalyzes the conversion of free IAA to IAA-aa, a portion of which (e.g. IAA-Ala, but not IAA-Trp) can be reconverted to free IAA. The expression levels of two PpyGH3 genes decreased from day 1 (Figure 5B). The expression levels of two PpyDAO genes encoding 2-oxoglutarate-dependent dioxygenase, which is essential for auxin catabolism and the maintenance of auxin homeostasis, were downregulated (Figure 5B). Furthermore, the expression of PpyIAA26, which encodes an Aux/IAA protein, decreased. The Aux/IAA proteins are repressors of the early auxin response genes at low auxin concentrations. Auxin transport genes (PpyPIN and PpyABCB) had upregulated expression levels, especially PpyPIN, which encode an auxin efflux carrier (Figure 5B). According to the RT-qPCR data (Supplemental Figure S8A), the expression of auxin biosynthesis genes (PpyYUCCA4-2 and PpyTAA1) and auxin transport genes (PpyPIN1b and PpyPIN1c) was upregulated, further verifying the changes in transcript levels. These results implied that during defoliation-induced flowering, auxin levels may be maintained because of an increase in the corresponding biosynthesis pathway and decreases in auxin catabolism.
In addition to auxin-related genes, the expression patterns of other hormone-related genes that influence autumn flowering along with auxin were also determined (Supplemental Figure S8B). The expression levels of a CK activation gene (PpyLOG1) and response gene (PpyARR) as well as a SL response inhibitor gene (PpySMXL) were upregulated. In contrast, an ABA response gene (PpyABI5) and an SL response gene (PpyBRC1) had downregulated expression levels. The changes in the expression of these genes matched the changes in the expression of auxin-related genes.
We also analyzed the expression patterns of PpyAG2, PpyCYCD3;1, and PpyEXP5, which are related to the development of pistil primordia as well as cell proliferation and expansion during flower bud break (Supplemental Figure S8B). The upregulated expression of these genes suggested that spur flower bud development and break were accelerated by defoliation.
In addition to pistil primordia development-related genes, we analyzed the expression of several bud transition-related genes that also regulate branching. There were two FT genes in pear, PpyFT1 and PpyFT2 (Supplemental Figure S9A). The expression level of PpyFT2 was considerably up-regulated after defoliation, whereas PpyFT1 did not substantially change (Supplemental Figure S9B). Furthermore, the expression levels of PpyTFL1-1, PpyTFL1-2, and PpyFLC remained stable (Supplemental Figure S9B). The transcriptional level of PpyFT downstream genes, PpyAP1 and PpyLAP1, was slightly reduced (Supplemental Figure S9B). These results revealed that PpyFT2 might play a role in regulating bud paradormancy release after defoliation.
A high auxin concentration inhibited flower bud break after defoliation
Because defoliation was accompanied by accelerated flower bud development and altered auxin distribution, we investigated whether high auxin concentrations can impede this process. Excised current-year mature shoots were sprayed with 300 mg/L 1-naphthaleneacetic acid (NAA) and then cultured in the same solution for 48 h. The bud break rate of the NAA-treated shoots was substantially lower than that of the control group (Figure 6A). New sprouts, including flowers and leaves, were detected in the control group at 20 days post-treatment, but there was no observable sign of bud break after the NAA treatment (Figure 6B). The SEM images indicated that flower bud development was inhibited by NAA (Figure 6, C and D). On day 5 after defoliation, the pistil primordia of the flower buds in the current-year long shoots were detectable (Figure 6C), but there was no obvious bulge in the center of the flower primordia after the NAA treatment (Figure 6D). We speculated a high auxin concentration can negatively regulate flower bud break by modulating the expression of these genes. The distribution and fluorescence intensity of PpyPIN1b in NAA-treated flower buds were visualized by immunohistochemistry (Supplemental Figure S10A). The fluorescence intensity of PpyPIN1b in the flower buds of the control group was enhanced about 1 day after defoliation, whereas there was no obvious change in the fluorescence intensity of PpyPIN1b in the flower buds treated with NAA after defoliation. A semi-quantitative analysis revealed a similar trend (Supplemental Figure S10B), indicating that the high auxin concentration might hinder auxin efflux from buds by inhibiting PpyPIN1b protein expression, thereby preventing flower bud break. We also verified the expression patterns of the previously identified DEGs following the NAA treatment. The expression trends of these genes were opposite to the corresponding expression trends following defoliation (Figure 6, E and F and Supplemental Figure S11). The expression of auxin metabolism, transport, and signaling genes was suppressed. The upregulated genes after defoliation were all downregulated after the NAA treatment. The genes that negatively regulate flower bud break were highly expressed. Accordingly, a high auxin concentration appears to be detrimental to flower bud break and disrupts the expression of genes in pathways involved in defoliation-induced flowering.
Figure 6.
Inhibitory effects of a high auxin concentration on flower bud break after defoliation and expression patterns of hormone-related genes after an NAA treatment. A, Bud break percentage of current-year long shoots after a 300 mg/L NAA treatment. B, Representative images of bud break on day 20 on defoliated (left) and NAA-treated current-year long shoots (right). Scale bars, 5 cm. C and D, Scanning electron micrographs of flower buds of current-year long shoots on day 5 in the control group (C) and the NAA-treated group (D). pp, petal primordium; triangles, stamen primordium; and asterisks, pistil primordium. E and F, Expression patterns of genes related to auxin (E) and other hormones (F) after the NAA treatment. Error bars indicate the SEs of three biological replicates and asterisks indicate significant differences between control (defoliated) and NAA-treated branches (Student’s t test). *P < 0.05, **P < 0.01, ***P < 0.001.
To further clarify the inhibitory effect of a high auxin concentration on bud flush, we sprayed “Cuiguan” pear trees with 300 mg/L NAA and observed that bud sprouting was inhibited (Supplemental Figure S12). Thus, treatments with high auxin concentrations may be useful for preventing out-of-season flowering after early defoliation.
Discussion
Leaves rather than apical dominance maintain bud paradormancy
In woody plant species, apical dominance is usually involved in paradormancy, which is typically released after decapitation, leading to lateral bud outgrowth (Hancock, 1960; Brown et al., 1967; Luckwill, 1968; Saure, 1985; Harmer, 1989; Dennis, 1994; Faust et al., 1997; Cline and Deppong, 1999). However, paradormancy is not only regulated by apical dominance. In some temperate woody species, decapitation is insufficient for inducing lateral bud break or it only induces the outgrowth of buds adjacent to the point of decapitation (Cline and Deppong, 1999). The weak response following decapitation suggests that factors unrelated to apical dominance repress lateral bud outgrowth. The leaf, stem, and fruit may have inhibitory effects on lateral bud break (Cozens and Wilkinson, 1966; Tinklin and Schwabe, 1970; Nagao, 1976; Champagnat, 1989; Haim et al., 2020). In rose plants, leaf and stem signals can inhibit the outgrowth of lateral buds (Zieslin and Halevy, 1976). These inhibitory factors and apical dominance may affect different growth and development periods. For example, lateral bud paradormancy is mainly controlled by apical dominance from March to May, whereas signals primarily from adult leaves maintain bud paradormancy from April to July and the stem plays a regulatory role from March to September (Champagnat, 1989). Thus, leaf compounds and signals might be crucial regulators of bud paradormancy during the summer and autumn. Because early defoliation of pear occurred in July–August and bud paradormancy was released after leaf abscission (Figure 2), leaves, but not apical dominance, might be the key regulator of this process. Similarly, defoliation in autumn promotes the reflowering of tree peony (Paeonia suffruticosa), a perennial woody plant (Xue et al., 2018; Wang et al., 2020). To increase its ornamental value, manual defoliation is often used to stimulate tree peony reflowering in autumn. This process involves the acceleration of flower bud morphological differentiation, as observed for autumn flowering in our study, but it also results in the induction of flower bud initiation. Although reflowering in peony is not exactly the same as early defoliation-induced autumn flowering in pear, both processes indicate that leaves are important for maintaining bud paradormancy.
In addition to being influenced by leaves, early defoliation-induced paradormancy release occurs in the lateral buds as well as the terminal buds, which differs from the effects of apical dominance. Specifically, apical dominance affects lateral buds and the maintenance of the paradormant state depends on auxin from the apex. During autumn flowering, spur buds as well as the apical and lateral buds in current-year long shoots can sprout. Spurs, which we focused on in this study, are fruiting structures unique to pears and apples and are the main flowering and fruit-bearing units (Huet, 1973; Forshey and Elfving, 1989; Petersen and Krost, 2013; De Alcântara Barbosa et al., 2018). Spur buds are apical buds, and their paradormancy is not controlled by apical dominance. Moreover, despite the apical dominance in current-year long shoots, in this study, both terminal and lateral buds sprouted after defoliation (e.g. control shoots in Figure 6B and Supplemental Figure S13D), meaning the presence of terminal buds did not inhibit the sprouting of lateral buds. Similarly, defoliation-induced apical bud burst was observed in apple (Taylor et al., 1984; Jones, 1987). Therefore, the inhibitory effect came from the leaves, but not the apical buds, during autumn flowering. Leaves are crucial for the regulation of pear bud paradormancy. However, the leaf components that regulate this process are unknown.
Auxin transport canalization from the bud to the stem promotes bud break accompanied by auxin transcriptional changes in the bud after early defoliation
In this study, many of the prominent transcript-level changes were to auxin-related genes (Figure 5; Supplemental Figures S7 and S8). In addition to photosynthetic products, mature leaves are a source of auxin. In pea, the IAA-synthesized de novo in mature leaves is used to maintain normal IAA levels throughout the plant via basipetal transport (Jager et al., 2007). Auxin is transported in the phloem to the lower stem and then into the roots. In pear, the auxin that accumulates in the wood contributes to bud break and the cambial reactivation after the cessation of shoot growth in winter, with mature leaves serving as an important auxin source (Yang et al., 1992). Additionally, auxin regulates leaf senescence and abscission. Blocked auxin transport in leaf veins can lead to leaf delamination and defoliation, implying that early defoliation decreases the availability of auxin produced in leaves (Jin et al., 2015). Thus, auxin from leaves might be involved in maintaining bud paradormancy.
A previous study demonstrated that IAA levels decrease in young and mature pea internodes after defoliation, similar to the stem auxin level after decapitation (Jager et al., 2007). However, in the current study, the IAA content in the flower bud stem increased after defoliation (Figure 4C). We speculated that a decrease in the stem IAA level due to defoliation may allow the efflux of IAA from buds, which subsequently replenishes the IAA in the stem at the base of the flower buds. The initiation of auxin transport canalization relies on relative differences in auxin levels between the bud and stem. During the regulation of branching, low stem auxin concentrations after the apex are removed to make the axillary buds and stem segments strong source and sink tissues, respectively, resulting in the flow of auxin from a source bud to a sink stem segment to release axillary buds (Bennett et al., 2006; Prusinkiewicz et al., 2009; Müller and Leyser, 2011; Walker and Bennett, 2018). Auxin efflux depends on the polar distribution of abundant PINs in the plasma membrane (Bennett et al., 2006, 2016a; Prusinkiewicz et al., 2009; Crawford et al., 2010; Müller and Leyser, 2011; Shinohara et al., 2013). Dormant buds retain large amounts of auxin, whereas activated buds have PIN auxin efflux carriers after decapitation to facilitate the polar auxin flow from buds (Qiu et al., 2019; Kotov et al., 2021). The auxin channels create a vascular connection between activated buds and stems, which is necessary for sustainable bud outgrowth (Balla et al., 2011). As predicted, the auxin efflux carrier PpyPIN1b tended to localize in the cell membrane after defoliation to accelerate auxin efflux from buds (Figure 4D). In addition to the changes in PpyPIN1b distribution, we focused on the expression-level alterations to auxin efflux carrier genes. We detected the upregulated expression of several PpyPIN and PpyABCB genes; this increase in expression was most notable for the PpyPIN genes (Figure 5B and Supplemental Figure S8A). In contrast, the application of exogenous NAA suppressed auxin transport from the bud to the stem. The RT-qPCR data and immunofluorescence results revealed that PpyPIN1b and the encoded protein expression were inhibited by the NAA treatment (Figure 6E and Supplemental Figure S10). These results indicated that defoliation induces auxin efflux from buds and alters auxin distribution, which influences bud paradormancy release. Unexpectedly, the changes in auxin efflux did not result in an obvious decrease in the bud auxin level (Figure 4, A and B and Supplemental Figure S2). However, this finding was similar to the change in auxin contents in buds released from apical dominance in some species, in which the auxin level remains constant or even increases during bud outgrowth (Thimann and Skoog, 1934; Sachs and Thimann, 1967; Thomas, 1972; Hillman et al., 1977; Jablanović and Nešković, 1977; Pilate et al., 1989; Gocal et al., 1991). For apical dominance, auxin synthesis increases in lateral buds after decapitation because of the negative feedback mechanism regulating auxin biosynthesis, especially the IPyA pathway (Suzuki et al., 2015; Takato et al., 2017). In the present study, the expression levels of auxin biosynthesis genes (PpyYUCCA and PpyTAA1) and genes (e.g. PpyMES17) encoding enzymes that convert conjugated auxin to the active form were upregulated (Figure 5B). In contrast, downregulated expression levels were detected for PpyGH3 and PpyDAO genes, which, respectively, encode enzymes catalyzing the conjugation of auxin to amino acids and the hydrolysis of IAA, indicative of a decrease in IAA conjugation and catabolism to maintain active IAA levels (Porco et al., 2016). On the basis of the observed minor alterations in the bud auxin level and the changes in the expression of auxin-related genes, we speculated that to maintain auxin homeostasis, auxin efflux stimulates auxin synthesis and the conversion of auxin conjugates, while also decreasing IAA catabolism.
Auxin promotes floral organ development (Cucinotta et al., 2020) and cell expansion (Du et al., 2020). A decrease in the auxin content was accompanied by a decrease in the transcriptional repressor AUX/IAA, which interacts with AUXIN REPONSE FACTORs (ARFs) to repress their function (Weijers and Wagner, 2016). The efflux of auxin might lead to an increase in the expression of specific genes, such as PpyARF3, as has been observed in strawberry (Qiu et al., 2019). Earlier research indicated that ARF3 can promote gynoecium morphogenesis via the AG pathway and cell wall changes (Liu et al., 2014; Andres-Robin et al., 2018). Consistent with the early development of pistil primordia, the PpyAG2 and PpyARF3 expression levels increased (Supplemental Figures S8B and S11A), implying auxin might further regulate bud outgrowth via its signal transduction pathway.
As expected, defoliation and the NAA treatment had the opposite effects on the expression of the DEGs related to auxin metabolism, transport, and signaling pathways (Figure 6E). The application of exogenous NAA inhibited PpyYUCCA and PpyTAA1 expression, which led to a decrease in auxin production, which is in accordance with the results of a previous study on Arabidopsis (Suzuki et al., 2015). Additionally, NAA inhibited the expression of PpyAG2 and PpyARF3, which adversely affected the maturation of floral organ primordia (Figure 6F and Supplemental Figure S11B).
These findings suggest that defoliation alters the auxin distribution and induces transcriptional modifications to induce blooming, which is inhibited by a high auxin concentration. In other words, auxin transport canalization from the bud to the stem is crucial for promoting early defoliation-induced bud break.
Auxin may co-regulate early defoliation-induced paradormancy release with other hormones
CK is a second messenger and its synthesis is suppressed by auxin. Although there were no major changes in flower bud CK contents after defoliation (Supplemental Figure S13A), the CK synthesis genes PpyLOG1 and PpyIPT3 were expressed at high levels in the buds and stems, respectively, to promote bud break after defoliation (Supplemental Figures S7, S8B, and S13B), similar to the expression-level changes in CK synthesis genes during the axillary bud outgrowth of strawberry runners (Qiu et al., 2019). We treated current-year long shoots with the CK synthesis inhibitor lovastatin and observed that bud break was inhibited (Supplemental Figure S13, C and D), implying that inhibited CK synthesis can hinder blooming. Additionally, CKs might be positively associated with high PpyPIN expression levels after defoliation. CKs promote PIN-mediated auxin efflux to promote branching in pea (Kalousek et al., 2010), Arabidopsis (Waldie and Leyser, 2018), and apple (Tan et al., 2019). Considering that PpyIPT1 expression was suppressed in buds after the NAA treatment (Supplemental Figure S13B), high auxin concentrations may hinder bud break by suppressing CK production and disrupting auxin efflux from buds.
Auxin and SL pathways are regulated by a feedback loop. More specifically, SL inhibits auxin synthesis (Ligerot et al., 2017; Zhang et al., 2020), whereas auxin increases SL synthesis, which inhibits branching (Sorefan et al., 2003; Foo et al., 2005; Johnson et al., 2006; Arite et al., 2007; Simons et al., 2007; Brewer et al., 2009; Walker and Bennett, 2018). SLs activate MAX2, which is a component of the Skp–Cullin–F-box complex that degrades SMAX1-LIKE6, 7, and 8 (SMXL6/7/8), resulting in the release of BRC1 and inhibited branching (Soundappan et al., 2015; Waters, 2017). In this study, SMXL expression levels were upregulated, whereas MAX2 expression was downregulated, which inhibited SL signal transduction (Supplemental Figures S7 and S8B), which were consistent with the changes observed during the axillary bud outgrowth of strawberry runners (Qiu et al., 2019). SL also interferes with PIN-dependent auxin transport via the MAX pathway (Bennett et al., 2006; Prusinkiewicz et al., 2009; Crawford et al., 2010; Shinohara et al., 2013; Zhang et al., 2020). The downregulated expression of PpyMAX2 and the upregulated expression of PpySMXL after defoliation (Supplemental Figures S7 and S8B) may help to enhance auxin efflux from buds to release bud paradormancy.
The BRC1 gene encodes a hub protein that integrates several regulatory signals to inhibit branching. Auxin and SL positively regulate BRC1 expression, whereas sugars, CK, and GA have the opposite effect (Rameau et al., 2015; Wang et al., 2019). Furthermore, BRC1 functions upstream of auxin to modulate PAT. In cucumber, CsBRC1 suppresses axillary bud outgrowth by inhibiting CsPIN3 expression (Shen et al., 2019). In this study, PpyBRC1 expression was downregulated after defoliation, but upregulated in response to a high auxin concentration (Supplemental Figures S7 and S8; Figure 6F). Thus, PpyBRC1 might integrate CK and SL to disrupt auxin efflux and inhibit autumn flowering.
Early defoliation-induced bud paradormancy release differs from FT-TFL1-LAP1 controlled floral transition and branching
The module consisting of FT, TFL1, and LAP1 regulates the floral transition and branching (Shannon and Meekswagner, 1991; Liljegren et al., 1999; Bohlenius et al., 2006; Hanano and Goto, 2011; Azeez et al., 2014; Moraes et al., 2019; Maurya et al., 2020a, 2020b; Singh et al., 2021). However, in this study, only the PpyFT2 expression level was substantially up-regulated after defoliation, while the PpyFT1, PpyTFL1-1, PpyTFL1-2, PpyAP1, PpyLAP1, and PpyFLC expression levels showed no substantial changes (Supplemental Figure S9B). In poplar, a high transcriptional level of FT2 in summer promotes vegetative growth and prevents premature growth cessation and bud set in autumn, whereas FT1 is required for reproductive onset and endodormancy release (Bohlenius et al., 2006; Miskolczi et al., 2019; Andre et al., 2022). Thus, we proposed that PpyFT2 was involved in the regulation of the growth of flower and leaf buds after defoliation, which was confirmed by the defoliation-advanced development of floral organ components, especially the early emergence of pistil primordia (Figure 3, B–O). These results suggest that early defoliation-induced bud breaking involves the acceleration of bud growth, differing from floral transition and branching regulated by the FT-TFL1-LAP1 module.
Conclusions
In this study, we revealed that altered auxin distribution induced by early defoliation promotes pear bud paradormancy release. In addition to being a source of energy, leaves may act through hormone pathways, especially auxin-related pathways (Figure 7). We proposed that leaves synthesize auxin, which is then transported to the stems that connect spur buds with a branch. Auxin accumulation in stems inhibits its efflux from buds, thereby maintaining paradormancy. The stem IAA content decreases when the supply of auxin decreases following early defoliation, allowing auxin efflux from the bud to promote bud paradormancy release. Changes to auxin distribution might also alleviate the inhibition of CK synthesis and hinder SL synthesis, thereby promoting bud paradormancy release by altering auxin transport or specific signal transduction pathways, with BRC1 as a hub protein. The application of high concentrations of auxin analogs inhibits bud break, which is associated with the restoration of leaf activities. Furthermore, because auxin can co-modulate apical dominance or branching with sugars and other hormones (Barbier et al., 2019), sugars may affect growth and development as signaling molecules. Thus, the relationships between sugars and auxin or other hormones will need to be investigated further to construct a more complete bud paradormancy regulatory network in woody fruit trees that differ from the apical dominance model. Taken together, the findings of this study may be useful for inhibiting early defoliation-induced out-of-season flowering in woody fruit trees to minimize yield losses.
Figure 7.
Proposed model of the physiological process of autumn flowering before (left) and after (right) defoliation. Decreased auxin contents in the stems at the base of flower buds after defoliation promote auxin efflux from buds, which activates flower buds. The effects of auxin combined with the stimulatory influence of CK and the diminished inhibitory effect of SL activate the PIN auxin efflux carrier and downregulate the expression of the branching inhibitor gene BRC1 to further break bud paradormancy. (Elements in the figure are not drawn to scale.)
Materials and methods
Plant materials and treatments
The artificial defoliation treatment was performed using early-ripening pear cultivar “Cuiguan” (Pyrus pyrifolia) trees, which are prone to early defoliation induced by disease. The trees were grown in Daqing Orchard, Fuyang district, Hangzhou city, Zhejiang province, China. Eighteen healthy adult trees with no defoliation were selected for the artificial defoliation treatment, six of which were used for calculating bud break rates. The remaining 12 trees were evenly divided into control and treatment groups, with two trees serving as one biological replicate. Approximately 70% of the leaves were manually removed on August 11, 2018, and August 5 from 2019 to 2020. Ten spurs per tree were selected to determine the bud break rate. The state of sprouting was assessed according to the appearance of fresh bud tissues and the subsequent growth. Spur flower buds and the stems at their base were sampled at 0, 1, 3, 5, 7, 10, 13, 16, and 20 days after defoliation, frozen in liquid nitrogen, and stored at −80°C until used.
Regarding the NAA treatment in the field, six “Cuiguan” pear trees that underwent the early defoliation treatment were selected in the experimental farm affiliated to Huzhou Academy of Agricultural Sciences in Wuxing district, Huzhou city, Zhejiang province, China, and then evenly divided into control and treatment groups. The control group was sprayed with water (containing 0.1% [v/v] Tween-20), whereas the treatment group was sprayed with 300 mg/L sodium naphthalene-1-acetate (NAA-Na) (containing 0.1% [v/v] Tween-20). For the NAA treatment in the laboratory, current-year branches (45 cm long) with full flower buds were collected from “Cuiguan” trees in Daqing Orchard. Leaves were removed from the control samples. After removing leaves from the branches, the treatment group was sprayed with 300 mg/L NAA-Na solution (containing 0.1% [v/v] Tween-20) until droplets were detectable on the surface of branches. The branches were then incubated in the same solution for 48 h in an artificial climate chamber before being transferred to clean water. The chamber conditions were as follows: 12-h light (25°C ± 1°C):12-h dark (21°C ± 1°C) cycle and 75% humidity. Bud break rates were calculated every 5 days and flower buds were collected at 0, 3, 5, 10, 15, and 20 days after initiating the incubation in clean water.
Paraffin sectioning and SEM
Flower buds after defoliation were fixed in 10% (w/v) formalin–acetic acid–alcohol solution and then embedded in paraffin and sectioned. The prepared slices were stained with safranin and solid green staining solution and then examined using the DM1000 LED microscope (Leica, Wetzlar, Germany) and photographed. Flower buds for the SEM analysis were fixed in a 4% (v/v) glutaraldehyde solution overnight at 4°C. They were then refixed in 1% (w/v) osmium acid for 1.5 h, dehydrated, and examined using the SU-8010 scanning electron microscope (Hitachi, Tokyo, Japan) (refer to the Supplemental Method for details).
Extraction of RNA and construction of the RNA-seq library
Flower buds from the defoliated and control groups were stripped of their external scales and immediately frozen in liquid nitrogen. Total RNA was extracted from flower buds using the RNAprep Pure Micro Kit (Tiangen, Beijing, China). Total RNA from flower buds collected at 0, 1, 3, 5, 7, and 10 days after defoliation were selected for the RNA-seq analysis, which was performed using three biological replicates per time-point. The library construction and sequencing were performed by Novogene (Beijing, China) on an Illumina Novaseq platform and 150 bp paired-end reads were generated. Total RNA was extracted from all other samples according to a modified cetyltrimethylammonium bromide method (Chang et al., 1993).
Analysis of DEGs
Clean data were obtained by eliminating low-quality reads and reads containing adapters or poly-N sequences from the raw sequence data (raw reads). The clean reads were aligned to the “Cuiguan” pear reference genome (Gao et al., 2021) using HISAT2 (https://daehwankimlab.github.io/hisat2/). The DEGs were identified with the DESeq2 R package (Love et al., 2014). A Benjamini–Hochberg-adjusted P-value < 0.05 was applied as the significance threshold. The KEGG pathway enrichment analysis was performed using TBtools (Chen et al., 2020). The DEGs were also functionally annotated and metabolic changes were analyzed using the MapMan program (version 3.6.0RC1) (http://mapman.gabipd.org/web/guest/mapmanstore) (Thimm et al., 2004).
Measurement of plant hormones in flower buds and stems
Phytohormone contents were determined by Shanghai Applied Protein Technology Co., Ltd. (APTBIO, Shanghai, China) using the AB Sciex QTRAP 5500 system as previously described (Shao et al., 2019) as well as by MetWare (Wuhan, China) (http://www.metware.cn/) using the 6500 LC-MS/MS platform. All analyses involved three replicates.
Immunofluorescence assay of IAA and PpyPIN1b
The IAA immunofluorescence localization method used in this study was a modified version of a published method (La Porta et al., 2019). Under vacuum conditions, flower buds were treated with a 4% 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/phosphate-buffered saline (1× PBS) (w/v) solution for 1 h at room temperature and then fixed in 4% (w/v) paraformaldehyde for 30 min. The localization of PpyPIN1b was performed according to a modified version of an established method (Paciorek et al., 2006). The flower buds used for the PpyPIN1b localization were fixed in 4% paraformaldehyde for 30 min at room temperature. All samples were dehydrated, embedded in wax, and sectioned. The sections were dewaxed, rehydrated, and then incubated in a solution comprising 10% (v/v) dimethyl sulfoxide, 3% Nonidet P-40, and 1× PBS for 30 min at room temperature. After blocking in 2% (w/v) bovine serum albumin (BSA) for 30 min at 37°C, the sections were incubated with anti-IAA antibody (1:600; Agrisera AS06 193) (Agrisera, Vännäs, Sweden) or anti-PpyPIN1b (1:100) (ABclonal Technology, Woburn, USA) in blocking solution overnight at 4°C. The PpyPIN1b cDNA fragment encoding an antigenic peptide (amino acids 336–496) was ligated into the bacterial expression vector pET-28a-SUMO using restriction sites BamHI/HindIII to express the recombinant peptide used to generate a PpyPIN1b-specific polyclonal antiserum. The primers used for plasmid construction are listed in Supplemental Table S1. Rabbits were immunized and the polyclonal antiserum was purified by affinity chromatography by ABclonal Technology (Wuhan, China). The specificity of the antibody was verified (Supplemental Figure S14). The sections were subsequently incubated with fluorescein (FITC)[AQ]-conjugated AffiniPure goat anti-rabbit IgG (H+L) (Proteintech, SA00003-2) (Proteintech, Chicago, USA) in a 2% (w/v) BSA solution for 1 h at room temperature. The sections were then stained with 2 mg/mL 4′,6-diamidino-2-phenylindole (DAPI, Sangon Biotech, E607303, Shanghai, China) for 10 min at room temperature before being examined using the A1plus confocal fluorescence microscope (Nikon, Tokyo, Japan). The excitation wavelength and the emission wavelength of DAPI were 405 and 400–490 nm, respectively. The excitation wavelength and the emission wavelength of FITC were 488 and 480–520 nm, respectively. The laser power of all channels was 2 mW. The gain parameter of all channels was set to 60. Specific details are provided in the Supplemental Method.
Validation of DEGs by a RT-qPCR analysis
Total RNA (1 μg) served as the template for the synthesis of cDNA using the PrimeScript RT reagent Kit with gDNA Eraser (TaKaRa, Tokyo, Japan). The RT-qPCR analysis was performed using the iTaq Universal SYBR Green Supermix (Bio-Rad) to validate the expression of DEGs as previously described (Tao et al., 2018). The RT-qPCR primers are listed in Supplemental Table S2 and Supplemental Table S3.
Phylogenetic analysis of FT family proteins
Multiple sequence alignment was performed with MUSCLE (Edgar, 2004). The spurious sequences or poorly aligned regions were removed from a multiple sequence alignment using trimAl (Capella-Gutierrez et al., 2009). The phylogenetic tree was constructed by the maximum-likelihood estimation method with IQ-TREE (Nguyen et al., 2015) and was displayed with Itol (Letunic and Bork, 2021). Bootstrap number was 5,000. The deduced amino acid sequences of members of the FT family were derived from pear (PpyFT1, EVM0026577; PpyFT2, EVM0021285), Arabidopsis (AtFT, AT1G65480.1), poplar (PtFT1, POPTR_0008s07730.1; PtFT2, POPTR_0010s18680.1), apple (MdFT1, AB161112; MdFT2, AB458504), peach (PpFT, EU939302), and grapevine (VvFT, DQ871590).
Statistical analysis
Data were subjected to a one-way ANOVA followed by Tukey’s multiple range test in the Statistical Product and Service Solutions program (Statistics 26) (SPSS Inc., Chicago, USA).
Accession numbers
The accession numbers for genes mentioned in this article are listed in Supplemental Tables S2–S5. Sequence data from this article can be found in the Genome Warehouse at the National Genomics Data Center, Beijing Institute of Genomics (China National Center for Bioinformation), Chinese Academy of Sciences, under accession numbers GWHBAOS00000000.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Trees after natural early defoliation and artificial defoliation.
Supplemental Figure S2. Immunofluorescence localization of IAA in flower buds after defoliation.
Supplemental Figure S3. Identification of DEGs and functional characterization via a KEGG pathway enrichment analysis.
Supplemental Figure S4. Sampling for RNA-seq analysis.
Supplemental Figure S5. DEGs related to energy metabolism (light reactions and sugar metabolism).
Supplemental Figure S6. Validation of RNA-seq data by RT-qPCR analysis.
Supplemental Figure S7. Heat map presenting the expression dynamics of key DEGs associated with nine hormones.
Supplemental Figure S8. Validation of hormone-related gene expression by RT-qPCR.
Supplemental Figure S9. Expression dynamics of key genes associated with floral transition and photoperiod- and temperature-regulated branching.
Supplemental Figure S10. Immunofluorescence localization of PpyPIN1b in flower buds after the treatment with a high auxin concentration.
Supplemental Figure S11. Relative expression of auxin- or GA-related genes after defoliation and the NAA treatment.
Supplemental Figure S12. A high concentration of exogenous auxin inhibits bud break after early defoliation in the field.
Supplemental Figure S13. Expression of genes involved in CK biosynthesis and the inhibition of bud break using the CK synthesis inhibitor lovastatin.
Supplemental Figure S14. Immunoblot for assessing anti-PpyPIN1b antibody specificity.
Supplemental Table S1. Primer sequences used for plasmid construction.
Supplemental Table S2. Primer sequences used in RT-qPCR experiments for validation of RNA-seq.
Supplemental Table S3. Primer sequences used in RT-qPCR experiments for hormone-related DEGs.
Supplemental Table S4. Annotation of DEGs related to sugar metabolism.
Supplemental Table S5. Annotation of hormone-related DEGs.
Funding
This work was supported by the China Agriculture Research System of MOF and MARA (CARS-28).
Conflict of interest statement. The authors declare that the publication of this paper has no clonfilicts of interest.
Supplementary Material
Contributor Information
Jia Wei, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Zhejiang Provincial Key Laboratory of Integrative Biology of Horticultural Plants, Hangzhou, 310058 Zhejiang, China; The Key Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Ministry of Agriculture of China, Hangzhou, 310058, Zhejiang, China.
Qinsong Yang, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Key Laboratory for Silviculture and Conservation, Ministry of Education, Beijing Forestry University, Haidian, Beijing 100083, China.
Junbei Ni, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Zhejiang Provincial Key Laboratory of Integrative Biology of Horticultural Plants, Hangzhou, 310058 Zhejiang, China; The Key Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Ministry of Agriculture of China, Hangzhou, 310058, Zhejiang, China.
Yuhao Gao, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Zhejiang Provincial Key Laboratory of Integrative Biology of Horticultural Plants, Hangzhou, 310058 Zhejiang, China; The Key Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Ministry of Agriculture of China, Hangzhou, 310058, Zhejiang, China.
Yinxin Tang, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Yantai Institute, China Agricultural University, Yantai, Shandong 264670, China.
Songling Bai, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Zhejiang Provincial Key Laboratory of Integrative Biology of Horticultural Plants, Hangzhou, 310058 Zhejiang, China; The Key Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Ministry of Agriculture of China, Hangzhou, 310058, Zhejiang, China.
Yuanwen Teng, College of Agriculture and Biotechnology, Zhejiang University, Hangzhou, Zhejiang 310058, China; Zhejiang Provincial Key Laboratory of Integrative Biology of Horticultural Plants, Hangzhou, 310058 Zhejiang, China; The Key Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Ministry of Agriculture of China, Hangzhou, 310058, Zhejiang, China; Hainan Institute of Zhejiang University, Sanya, Hainan 572000, China.
J.W., S.B., and Y.Teng designed the study, analyzed the data, and drafted the manuscript. J.W. conducted most of the experiments. J.W., S.B., and Q.Y. analyzed the RNA-seq data. J.N., Y.G., and Y.Tang completed some of the experiments.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is: Yuanwen Teng (ywteng@zju.edu.cn).
References
- Aguilar-Martínez JA, Poza-Carrión C, Cubas P (2007) Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 19: 458–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre D, Marcon A, Lee KC, Goretti D, Zhang B, Delhomme N, Schmid M, Nilsson O (2022) FLOWERING LOCUS T paralogs control the annual growth cycle in Populus trees. Curr Biol 32: 1–9 [DOI] [PubMed] [Google Scholar]
- Andres-Robin A, Reymond MC, Dupire A, Battu V, Dubrulle N, Mouille G, Lefebvre V, Pelloux J, Boudaoud A, Traas J, et al. (2018) Evidence for the regulation of gynoecium morphogenesis by ETTIN via cell wall dynamics. Plant Physiol 178: 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51: 1019–1029 [DOI] [PubMed] [Google Scholar]
- Azeez A, Miskolczi P, Tylewicz S, Bhalerao RP (2014) A tree ortholog of APETALA1 mediates photoperiodic control of seasonal growth. Curr Biol 24: 717–724 [DOI] [PubMed] [Google Scholar]
- Balla J, Kalousek P, Reinöhl V, Friml J, Procházka S (2011) Competitive canalization of PIN-dependent auxin flow from axillary buds controls pea bud outgrowth. Plant J 65: 571–577 [DOI] [PubMed] [Google Scholar]
- Bangerth F (1989) Dominance among fruits/sinks and the search for a correlative signal. Physiol Plant 76: 608–614 [Google Scholar]
- Banno K, Hayashi S, Tanabe K (1986) Morphological and histological studies on flower bud differentiation and development in Japanese pear (Pyrus serotina Rehd.). J Jap Soc Horticult Sci 55: 258–265 [Google Scholar]
- Barbier FF, Dun EA, Kerr SC, Chabikwa TG, Beveridge CA (2019) An update on the signals controlling shoot branching. Trends Plant Sci 24: 220–236 [DOI] [PubMed] [Google Scholar]
- Bennett T, Hines G, van Rongen M, Waldie T, Sawchuk MG, Scarpella E, Ljung K, Leyser O (2016a) Connective auxin transport in the shoot facilitates communication between shoot apices. PLoS Biol 14: e1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Liang Y, Seale M, Ward S, Müller D, Leyser O (2016b) Strigolactone regulates shoot development through a core signalling pathway. Biol Open 5: 1806–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16: 553–563 [DOI] [PubMed] [Google Scholar]
- Bohlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Booker J, Chatfield S, Leyser O (2003) Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell 15: 495–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun N, Germain AS, Pillot JP, Boutet-Mercey S, Dalmais M, Antoniadi I, Li X, Maia-Grondard A, le Signor C, Bouteiller N, et al. (2012) The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol 158: 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CL, McAlpine RG, Kormanik PP (1967) Apical dominance and form in woody plants: a reappraisal. Am J Bot 54: 153–162 [Google Scholar]
- Capella-Gutierrez S, Silla-Martinez JM, Gabaldon T (2009) trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25: 1972–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat P (1989) Rest and activity in vegetative buds of trees. Ann Forest Sci 46: 9–26 [Google Scholar]
- Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11: 113–116 [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13: 1194–1202 [DOI] [PubMed] [Google Scholar]
- Cline MG, Deppong DO (1999) The role of apical dominance in paradormancy of temperate woody plants: a reappraisal. J Plant Physiol 155: 350–356 [Google Scholar]
- Costa G, Ramina A (2014) Temperate fruit species. InDixon GR, Aldous DE, eds, Horticulture: Plants for People and Places, Volume 1: Production Horticulture, Springer Netherlands, Dordrecht, pp 97–121 [Google Scholar]
- Cozens IG, Wilkinson EH (1966) Control of lateral bud inhibition, flower emergence and dormancy in the blackcurrant. Nature 211: 867–868 [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- Cucinotta M, Cavalleri A, Chandler JW, Colombo L (2020) Auxin and flower development: a blossoming field. Cold Spring Harb Perspect Biol 13: a039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Alcântara Barbosa CM, Pio R, de Souza FBM, Bisi RB, Bettiol Neto JE, da Hora Farias D (2018) Phenological evaluation for determination of pruning strategies on pear trees in the tropics. Sci Horticult 240: 326–332 [Google Scholar]
- Dennis FG (1994) Dormancy—what we know (and don’t know). HortScience 29: 1249–1255 [Google Scholar]
- Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Du M, Spalding EP, Gray WM (2020) Rapid auxin-mediated cell expansion. Annu Rev Plant Biol 71: 5.1–5.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun EA, Germain AS, Rameau C, Beveridge CA (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158: 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC (2004) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform 5: 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust M, Erez A, Rowland LJ, Wang SY, Norman HA (1997) Bud dormancy in perennial fruit trees: physiological basis for dormancy induction, maintenance, and release. HortScience 32: 623–629 [Google Scholar]
- Finlayson SA (2007) Arabidopsis TEOSINTE BRANCHED1-LIKE 1 regulates axillary bud outgrowth and is homologous to monocot TEOSINTE BRANCHED1. Plant Cell Physiol 48: 667–677 [DOI] [PubMed] [Google Scholar]
- Foo E, Bullier E, Goussot M, Foucher F, Rameau C, Beveridge CA (2005) The branching gene RAMOSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17: 464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forshey CG, Elfving DC (1989) The relationship between vegetative growth and fruiting in apple trees. Horticult Rev 11: 229–287 [Google Scholar]
- Gao Y, Yang Q, Yan X, Wu X, Yang F, Li J, Wei J, Ni J, Ahmad M, Bai S, et al. (2021) High-quality genome assembly of ‘Cuiguan’ pear (Pyrus pyrifolia) as a reference genome for identifying regulatory genes and epigenetic modifications responsible for bud dormancy. Horticult Res 8: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q, Dai J, Jie B, Zhong S, Wang H, Wang W-C (2011) Advances in first bloom dates and increased occurrences of yearly second blooms in eastern China since the 1960s: further phenological evidence of climate warming. Ecol Res 26: 713–723 [Google Scholar]
- Gocal GFW, Pharis RP, Yeung EC, Pearce D (1991) Changes after decapitation in concentrations of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv Tender Green. Plant Physiol 95: 344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Rabinovich M, Smith HM (2021) The role of auxin and sugar signaling in dominance inhibition of inflorescence growth by fruit load. Plant Physiol 187: 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot J-P, Letisse F, Matusova R, Danoun S, Portais J-C, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Guan JC, Koch KE, Suzuki M, Wu S, Latshaw S, Petruff T, Goulet C, Klee HJ, Mccarty DR (2012) Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiol 160: 1303–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim D, Shalom L, Simhon Y, Shlizerman L, Kamara I, Morozov M, Albacete A, Rivero RM, Sadka A (2020) Alternate bearing in fruit trees: fruit presence induces polar auxin transport in citrus and olive stem and represses IAA release from the bud. J Exp Bot 72: 2450–2462 [DOI] [PubMed] [Google Scholar]
- Hall SM, Hillman JR (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. Timing of bud growth following decapitation. Planta 123: 137–143 [DOI] [PubMed] [Google Scholar]
- Hanano S, Goto K (2011) Arabidopsis TERMINAL FLOWER1 Is involved in the regulation of flowering time and inflorescence development through transcriptional repression. Plant Cell 23: 3172–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock H (1960) The experimental modification of branch form in an apple rootstock. Bot Gazette 121: 208–215 [Google Scholar]
- Harmer R (1989) The effect of mineral nutrients on growth, flushing, apical dominance and branching in Quercus petraea ( Matt.) Liebl. Forestry 62: 383–396 [Google Scholar]
- Hillman JR, Math VB, Medlow GC (1977) Apical dominance and the levels of indole acetic acid in Phaseolus lateral buds. Planta 134: 191–193 [DOI] [PubMed] [Google Scholar]
- Huang X, Lin J, Chen X, Zhang C, Zeng S, Chen C, Hu N (2015) Correlation analysis between pear leaf diseases and early-senescence and defoliation. Plant Protect 41: 160–164,183 [Google Scholar]
- Huet J (1973) Floral initiation in pear trees. Acta Horticult 34: 193–198 [Google Scholar]
- Iwata H, Gaston A, Remay A, Thouroude T, Jeauffre J, Kawamura K, Oyant LH-S, Araki T, Denoyes B, Foucher F (2012) The TFL1 homologue KSN is a regulator of continuous flowering in rose and strawberry. Plant J 69: 116–125 [DOI] [PubMed] [Google Scholar]
- Jablanović M, Nešković M (1977) Changes in endogenous level of auxins and cytokinins in axillary buds of pisutn sativum L. in relation to apical dominance. Biol Plant 19: 34–39 [Google Scholar]
- Jager CE, Symons GM, Glancy NE, Reid JB, Ross JJ (2007) Evidence that the mature leaves contribute auxin to the immature tissues of pea (Pisum sativum L.). Planta 226: 361–368 [DOI] [PubMed] [Google Scholar]
- Jin X, Zimmermann J, Polle A, Fischer U (2015) Auxin is a long-range signal that acts independently of ethylene signaling on leaf abscission in Populus. Front Plant Sci 6: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson X, Brcich T, Dun EA, Goussot M, Haurogné K, Beveridge CA, Rameau C (2006) Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol 142: 1014–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HG (1987) Repeat flowering in apple caused by water stress or defoliation. Trees 1: 135–138 [Google Scholar]
- Kalousek P, Buchtová D, Balla J, Reinöhl V, Procházka S (2010) Cytokinins and polar transport of auxin in axillary pea buds. Acta Univ Agric Silvic Mendelianae Brun 58: 79–88 [Google Scholar]
- Kotov AA, Kotova LM, Romanov GA (2021) Signaling network regulating plant branching: recent advances and new challenges. Plant Sci 307: 110880. [DOI] [PubMed] [Google Scholar]
- La Porta CAM, Lionetti MC, Bonfanti S, Milan S, Ferrario C, Rayneau-Kirkhope D, Beretta M, Hanifpour M, Fascio U, Ascagni M, et al. (2019) Metamaterial architecture from a self-shaping carnivorous plant. Proc Natl Acad Sci USA 116: 18777–18782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang GA, Early JD, Martin GC, Darnell RL (1987) Endodormancy, paradormancy, and ecodormancy—physiological terminology and classification for dormancy research. HortScience 22: 371–377 [Google Scholar]
- Letunic I, Bork P (2021) Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49: W293–W296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-J, Bangerth F (1999) Autoinhibition of indoleacetic acid transport in the shoots of two-branched pea (Pisum sativum) plants and its relationship to correlative dominance. Physiol Plant 106: 415–420 [Google Scholar]
- Ligerot Y, de Saint Germain A, Waldie T, Troadec C, Citerne S, Kadakia N, Pillot JP, Prigge M, Aubert G, Bendahmane A, et al. (2017) The pea branching RMS2 gene encodes the PsAFB4/5 auxin receptor and is involved in an auxin-strigolactone regulation loop. PLoS Genet 13: e1007089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljegren SJ, Gustafson-Brown C, Pinyopich A, Ditta GS, Yanofsky MF (1999) Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 11: 1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dinh TT, Li D, Shi B, Li Y, Cao X, Guo L, Pan Y, Jiao Y, Chen X (2014) AUXIN RESPONSE FACTOR 3 integrates the functions of AGAMOUS and APETALA2 in floral meristem determinacy. Plant J 80: 629–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckwill LC (1968) The effect of certain growth regulators on growth and apical dominance of young apple trees. J Horticult Sci 43: 91–101 [Google Scholar]
- Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya JP, Miskolczi PC, Mishra S, Singh RK, Bhalerao RP (2020a) A genetic framework for regulation and seasonal adaptation of shoot architecture in hybrid aspen. Proc Natl Acad Sci USA 117: 11523–11530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya JP, Singh RK, Miskolczi PC, Prasad AN, Jonsson K, Wu F, Bhalerao RP (2020b) Branching regulator BRC1 mediates photoperiodic control of seasonal growth in hybrid aspen. Curr Biol 30: 122–126.e122 [DOI] [PubMed] [Google Scholar]
- Minakuchi K, Kameoka H, Yasuno N, Umehara M, Luo L, Kobayashi K, Hanada A, Ueno K, Asami T, Yamaguchi S, et al. (2010) FINE CULM1 (FC1) works downstream of strigolactones to inhibit the outgrowth of axillary buds in rice. Plant Cell Physiol 51: 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskolczi P, Singh RK, Tylewicz S, Azeez A, Maurya JP, Tarkowska D, Novak O, Jonsson K, Bhalerao RP (2019) Long-range mobile signals mediate seasonal control of shoot growth. Proc Natl Acad Sci USA 116: 10852–10857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes TS, Dornelas MC, Martinelli AP (2019) FT/TFL1: calibrating plant architecture. Front Plant Sci 10: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Leyser O (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot 107: 1203–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D, Waldie T, Miyawaki K, To JPC, Melnyk CW, Kieber JJ, Kakimoto T, Leyser O (2015) Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J 82: 874–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao R (1976) Lateral bud outgrowth and its control by the apex. Bot Rev 42: 83–113 [Google Scholar]
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ (2015) IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32: 268–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, Sauer M, Balla J, Wiśniewska J, Friml J (2006) Immunocytochemical technique for protein localization in sections of plant tissues. Nat Protoc 1: 104–107 [DOI] [PubMed] [Google Scholar]
- Patrick JW, Botha FC, Birch RG (2013) Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol J 11: 142–156 [DOI] [PubMed] [Google Scholar]
- Petersen R, Krost C (2013) Tracing a key player in the regulation of plant architecture: the columnar growth habit of apple trees (Malus × domestica). Planta 238: 1–22 [DOI] [PubMed] [Google Scholar]
- Pilate G, Sossountzov L, Miginiac E (1989) Hormone levels and apical dominance in the aquatic fern marsilea drummondii A. Br. Plant Physiol 90: 907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porco S, Pěnčík A, Rashed A, Voß U, Casanova-Sáez R, Bishopp A, Golebiowska A, Bhosale R, Swarup R, Swarup K, et al. (2016) Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc Natl Acad Sci USA 113: 11016–11021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad TK, Li X, Abdel-Rahman AM, Hosokawa Z, Cloud NP, Lamotte CE, Cline MG (1993) Does auxin play a role in the release of apical dominance by shoot inversion in Ipomoea nil? Ann Bot 71: 223–229 [Google Scholar]
- Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Guan SC, Wen C, Li P, Gao Z, Chen X (2019) Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol 19: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameau C, Bertheloot J, Leduc N, Andrieu B, Foucher F, Sakr S (2015) Multiple pathways regulate shoot branching. Front Plant Sci 5: 741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T, Thimann KV (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54: 136–144 [Google Scholar]
- Saure MC (1985) Dormancy release in deciduous fruit trees. Horticult Rev 7: 239–300 [Google Scholar]
- Seale M, Bennett T, Leyser O (2017) BRC1 expression regulates bud activation potential, but is not necessary or sufficient for bud growth inhibition in Arabidopsis. Development 144: 1661–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon S, Meekswagner DR (1991) A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3: 877–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Zhou H-Z, Wu Y, Zhang H, Lin J, Jiang X, He Q, Zhu J, Li Y, Yu H, et al. (2019) OsSPL3, an SBP-domain protein, regulates crown root development in rice. Plant Cell 31: 1257–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Zhang Y, Ge D, Wang Z, Song W, Gu R, Che G, Cheng Z, Liu R, Zhang X (2019) CsBRC1 inhibits axillary bud outgrowth by directly repressing the auxin efflux carrier CsPIN3 in cucumber. Proc Natl Acad Sci USA 116: 17105–17114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin–cytokinin interactions in the control of shoot branching. Plant Mol Biol 69: 429–435 [DOI] [PubMed] [Google Scholar]
- Shinohara N, Taylor C, Leyser O, Scheres B (2013) Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol 11: e1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JL, Napoli CA, Janssen BJ, Plummer KM, Snowden KC (2007) Analysis of the DECREASED APICAL DOMINANCE genes of petunia in the control of axillary branching. Plant Physiol 143: 697–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RK, Bhalerao RP, Maurya JP (2021) When to branch: seasonal control of shoot architecture in trees. FEBS J https://doi.org/10.1111/febs.16227 [DOI] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Leyser O (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17: 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundappan I, Bennett T, Morffy N, Liang Y, Stanga JP, Abbas A, Leyser O, Nelson DC (2015) SMAX1-LIKE/D53 family members enable distinct MAX2-dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell 27: 3143–3159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Yamazaki C, Mitsui M, Kakei Y, Mitani Y, Nakamura A, Ishii T, Soeno K, Shimada Y (2015) Transcriptional feedback regulation of YUCCA genes in response to auxin levels in Arabidopsis. Plant Cell Rep 34: 1343–1352 [DOI] [PubMed] [Google Scholar]
- Takato S, Kakei Y, Mitsui M, Ishida Y, Suzuki M, Yamazaki C, Hayashi K, Ishii T, Nakamura A, Soeno K, et al. (2017) Auxin signaling through SCFTIR1/AFBs mediates feedback regulation of IAA biosynthesis. Biosci Biotechnol Biochem 81: 1320–1326 [DOI] [PubMed] [Google Scholar]
- Tan M, Li G, Chen X, Xing L, Ma J, Zhang D, Ge H, Han M, Sha G, An N (2019) Role of cytokinin, strigolactone, and auxin export on outgrowth of axillary buds in apple. Front Plant Sci 10: 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45: 1028–1036 [DOI] [PubMed] [Google Scholar]
- Tao R, Bai S, Ni J, Yang Q, Zhao Y, Teng Y (2018) The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta 248: 37–48 [DOI] [PubMed] [Google Scholar]
- Taylor JS, Pharis RP, Loveys B, Notodimedjo S, Edwards GR (1984) Changes in endogenous hormones in apple during bud burst induced by defoliation. Plant Growth Regul 2: 117–134 [Google Scholar]
- Thimann KV, Skoog F (1934) On the inhibition of bud development and other functions of growth substance in Vicia Faba. Proc R Soc B Biol Sci 114: 317–339 [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37: 914–939 [DOI] [PubMed] [Google Scholar]
- Thomas TH (1972) The distribution of hormones in relation to apical dominance in Brussels sprouts (Brassica oleracea var. gemmifera L.) plants. J Exp Bot 23: 294–301 [Google Scholar]
- Tinklin IG, Schwabe WW (1970) Lateral bud dormancy in the blackcurrant Ribes nigrum (L.). Ann Bot 34: 691–706 [Google Scholar]
- Tromp J (2000) Flower-bud formation in pome fruits as affected by fruit thinning. Plant Growth Regul 31: 27–34 [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Waldie T, Leyser O (2018) Cytokinin targets auxin transport to promote shoot branching. Plant Physiol 177: 803–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CH, Bennett T (2018) Forbidden fruit: dominance relationships and the control of shoot architecture. Annu Plant Rev 1: 1–38 [Google Scholar]
- Wang JJ, Wang QH (2009) Preliminary report on the phenomenon of second flowering of woody plant. J Gansu Forestry Sci Technol 34: 10–15, 59 [Google Scholar]
- Wang M, Le Moigne M-A, Bertheloot J, Crespel L, Perez-Garcia M-D, Ogé L, Demotes-Mainard S, Hamama L, Davière J-M, Sakr S (2019) BRANCHED1: a key hub of shoot branching. Front Plant Sci 10: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang S, Xue Y, Ren X, Xue J, Zhang X (2020) Defoliation, not gibberellin, induces tree peony autumn reflowering regulated by carbon allocation and metabolism in buds and leaves. Plant Physiol Biochem 151: 545–555 [DOI] [PubMed] [Google Scholar]
- Waters MT (2017) Strigolactones and karrikins. InThomas B, Murray BG, Murphy DJ, eds, Encyclopedia of Applied Plant Sciences, Ed 2, Vol 1. Academic Press, Oxford, pp 466–472 [Google Scholar]
- Weijers D, Wagner D (2016) Transcriptional responses to the auxin hormone. Annu Rev Plant Biol 67: 539–574 [DOI] [PubMed] [Google Scholar]
- Wilkie JD, Sedgley M, Olesen T (2008) Regulation of floral initiation in horticultural trees. J Exp Bot 59: 3215–3228 [DOI] [PubMed] [Google Scholar]
- Xue J, Li T, Wang S, Xue Y, Hu F, Zhang X (2018) Elucidation of the mechanism of reflowering in tree peony (Paeonia suffruticosa) ‘Zi Luo Lan’ by defoliation and gibberellic acid application. Plant Physiol Biochem 132: 571–578 [DOI] [PubMed] [Google Scholar]
- Yang HM, Ozaki T, Ichii T, Nakanishi T, Kawai Y (1992) Diffusible and extractable auxins in young Japanese pear trees. Sci Horticult 51: 97–106 [Google Scholar]
- Zhang J, Mazur E, Balla J, Gallei M, Kalousek P, Medveďová Z, Li Y, Wang Y, Prát T, Vasileva M, et al. (2020) Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat Commun 11: 3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QJ, Zhong BF, Li WG, Deng JL, Zhang SL (2016) Variation of organic nutrition on shoot and flower bud of ‘Hosui’ pear (Pyrus pyrifolia Nakai) during reflorescence period. Acta Bot Boreal-Occid Sin 36: 493–498 [Google Scholar]
- Zhao ZG, Huang NZ, Tang FL, Fu CM, Shi YP, Huang ZQ (2011) Research on the cause of physiologic abnormity of south-early-ripening-pear in North Guangxi. Guangxi Sci 18: 298–303 [Google Scholar]
- Zhu Y, Klasfeld S, Jeong CW, Jin R, Goto K, Yamaguchi N, Wagner D (2020) TERMINAL FLOWER 1-FD complex target genes and competition with FLOWERING LOCUS T. Nat Commun 11: 5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieslin N, Halevy A (1976) Components of axillary bud inhibition in rose plants. I. The effect of different plant parts (correlative inhibition). Bot Gaz 137: 291–296 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.